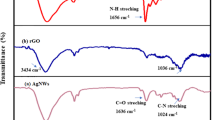
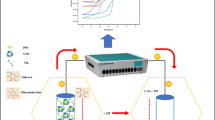
Abstract
As enzyme-immobilization and electron-transfer are the key factors for fabricating an enzymatic bioelectrode and its devices, we investigated a strategy to simultaneously improve the two aspects by assistance of the cationic surfactant, stearyltrimethylammonium bromide (STAB). By electrodeposition method, we obtained a multifunctional STAB-modified nanomaterial on electrode surface to improve enzyme-immobilization and electron-transfer. On the one hand, STAB could firmly adsorb a substantial number of enzymes via electrostatic interaction in a favorable orientation on the conductive nanomaterial surface for electron-transfer. On the other hand, STAB acts as a dispersant and stabilizer for traditionally conductive nanomaterials (reduced graphene oxide, carbon nanotubes, and gold nanoparticles) to guarantee their unique properties and form a well-conductive network. Electrochemical measurements demonstrated that enzymatic electrodes based on the nanohybrid possessed fast electron-transfer rate, a large quantity of immobilized enzymes, and good activity toward glucose oxidation or oxygen reduction. The glucose biosensor performed linear response range of 0.01–11.71 mM, detection limit of 3.84 × 10−3 mM, and sensitivity of 10.42 μA mM−1 cm−2, while the glucose/O2 biofuel cell exhibited maximum power density of 121.87 μW cm−2 and open-circuit voltage of 0.663 V. Both of the devices showed better performances than those of devices without STAB or conductive nanomaterials in this work.

Fabrication of surfactant-modified enzymatic electrode and its electrochemical performance










Similar content being viewed by others
References
Reid RC, Jones SR, Hickey DP, Minteer SD, Gale BK (2016) Modeling carbon nanotube connectivity and surface activity in a contact lens biofuel cell. Electrochim Acta 203:30–40
Cadet M, Gounel S, Stines-Chaumeil C, Brilland X, Rouhana J, Louerat F, Mano N (2016) An enzymatic glucose/O2 biofuel cell operating in human blood. Biosens Bioelectron 83:60–67
Slaughter G, Kulkarni T (2016) A self-powered glucose biosensing system. Biosens Bioelectron 78:45–50
Hickey DP, Reid RC, Milton RD, Minteer SD (2016) A self-powered amperometric lactate biosensor based on lactate oxidase immobilized in dimethylferrocene-modified LPEI. Biosens Bioelectron 77:26–31
Zhang EH, Xie Y, Ci SQ, Jia JC, Wen ZH (2016) Porous Co3O4 hollow nanododecahedra for nonenzymatic glucose biosensor and biofuel cell. Biosens Bioelectron 81:46–53
Hu CY, Yang DP, Zhu FJ, Jiang FJ, Shen SY, Zhang JL (2014) Enzyme-labeled Pt@BSA nanocomposite as a facile electrochemical biosensing interface for sensitive glucose determination. ACS Appl Mater & Inter 6(6):4170–4178
Wey TA, Southcott M, Jemison WD, MacVittie K, Katz E (2014) Electrical circuit model and dynamic analysis of implantable enzymatic biofuel cells operating in vivo. P IEEE 102(11):1795–1810
Qu FJ, Ma XY, Hui YC, Hou XZ, Yu J, Zhang QL, Chen F (2015) Fabrication of dual-path electron transfer electrode for electrochemical glucose sensing. J Electrochem Soc 162(1):B27–B35
Wooten M, Karra S, Zhang MG, Gorski W (2014) On the direct electron transfer, sensing, and enzyme activity in the glucose oxidase/carbon nanotubes system. Anal Chem 86(1):752–757
Petkova GA, Zaruba CK, Zvatora P, Kral V (2012) Gold and silver nanoparticles for biomolecule immobilization and enzymatic catalysis. Nanoscale Res Lett 7(1):287
Li Y, Huang XR, Qu YB (2013) A strategy for efficient immobilization of laccase and horseradish peroxidase on single-walled carbon nanotubes. J Chem Technol Biot 88(12):2227–2232
Ma S, Mu J, Qu Y, Jiang L (2009) Effect of refluxed silver nanoparticles on inhibition and enhancement of enzymatic activity of glucose oxidase. Colloid Surface A 345(1–3):101–105
Walcarius A, Minteer SD, Wang J, Lin YH, Merkoci A (2013) Nanomaterials for bio-functionalized electrodes: recent trends. J Mater Chem B 1(38):4878–4908
Wang ZG, Wang Y, Xu H, Li G, Xu ZK (2009) Carbon nanotube-filled nanofibrous membranes electrospun from poly(acrylonitrile-co-acrylic acid) for glucose biosensor. J Phys Chem C 113(7):2955–2960
Babadi AA, Bagheri S, Hamid SBA (2016) Progress on implantable biofuel cell: Nano-carbon functionalization for enzyme immobilization enhancement. Biosens Bioelectron 79:850–860
Godman NP, DeLuca JL, McCollum SR, Schmidtke DW, Glatzhofer DT (2016) Electrochemical characterization of layer-by-layer assembled ferrocene-modified linear poly(ethylenimine)/enzyme bioanodes for glucose sensor and biofuel cell applications. Langmuir 32(14):3541–3551
Vivekananthan J, Rincon RA, Kuznetsov V, Poller S, Schuhmann W (2014) Biofuel-cell cathodes based on bilirubin oxidase immobilized through organic linkers on 3D hierarchically structured carbon electrodes. Chemelectrochem 1(11):1901–1908
Najafi-Taher R, Derakhshan MA, Faridi-Majidi R, Amani A (2015) Preparation of an ascorbic acid/PVA-chitosan electrospun mat: a core/shell transdermal delivery system. RSC Adv 5(62):50462–50469
Moreno-Cortez IE, Romero-Garcia J, Gonzalez-Gonzalez V, Garcia-Gutierrez DI, Garza-Navarro MA, Cruz-Silva R (2015) Encapsulation and immobilization of papain in electrospun nanofibrous membranes of PVA cross-linked with glutaraldehyde vapor. Mat Sci Eng C-Mater 52:306–314
Zhao M, Gao Y, Sun JY, Gao F (2015) Mediatorless glucose biosensor and direct electron transfer type glucose/air biofuel cell enabled with carbon nanodots. Anal Chem 87(5):2615–2622
Korani A, Salimi A, Hadadzadeh H (2015) Nickel-phendione complex covalently attached onto carbon nanotube/cross linked glucose dehydrogenase as bioanode for glucose/oxygen compartment-less biofuel cell. J Power Sources 282:586–595
Kwon KY, Kim JH, Youn J, Jeon C, Lee J, Hyeon T, Park HG, Chang HN, Kwon Y, Ha S, Jung HT, Kim J (2014) Electrochemical activity studies of glucose oxidase (GOx)-based and pyranose oxidase (POx)-based electrodes in mesoporous carbon: toward biosensor and biofuel cell applications. Electroanal 26(10):2075–2079
Wen D, Liu W, Herrmann AK, Eychmuller A (2014) A membraneless glucose/O2 biofuel cell based on Pd aerogels. Chem-Eur J 20(15):4380–4385
Umasankar Y, Ramasamy RP (2014) Enhanced electron transfer in enzymatic bioelectrodes by a poly(vinyl alcohol) N-methyl-4(4′-formylstyryl) pyridinium methosulfate acetal cationic polymer. Chemelectrochem 1(11):1834–1839
Neto SA, Almeida TS, Belnap DM, Minteer SD, De Andrade AR (2015) Enhanced reduced nicotinamide adenine dinucleotide electrocatalysis onto multi-walled carbon nanotubes-decorated gold nanoparticles and their use in hybrid biofuel cell. J Power Sources 273:1065–1072
Yu YY, Chen ZG, He SJ, Zhang BB, Li XC, Yao MC (2014) Direct electron transfer of glucose oxidase and biosensing for glucose based on PDDA-capped gold nanoparticle modified graphene/multi-walled carbon nanotubes electrode. Biosens Bioelectron 52:147–152
Moumene M, Tabet-Aoul A, Gougis M, Rochefort D, Mohamedi M (2014) Laser pulse deposited nanosized ceria for direct electron transfer of glucose oxidase. Int J Electrochem Sc 9(1):176–184
Rajbongshi J, Das DK, Mazumdar S (2010) Direct electrochemistry of dinuclear Cu-a fragment from cytochrome c oxidase of Thermus thermophilus at surfactant modified glassy carbon electrode. Electrochim Acta 55(13):4174–4179
Wang F, Chen X, Xu Y, Hu S, Gao Z (2007) Enhanced electron transfer for hemoglobin entrapped in a cationic gemini surfactant films on electrode and the fabrication of nitric oxide biosensor. Biosens Bioelectron 23(2):176–182
Rusling JF, Nassar AEF (1993) Enhanced electron-transfer for myoglobin in surfactant films on electrodes. J Am Chem Soc 115(25):11891–11897
Hamachi I, Noda S, Kunitake T (1991) Functional conversion of myoglobin bound to synthetic bilayer membranes: from dioxygen storage protein to redox enzyme. J Am Chem Soc 113(25):9625–9630
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80(6):1339–1339
Guan HN, Wang W, Liu XF, Liang JZ (2014) Real-time visualization of colorimetric probe for pH-sensitive based on poly-(gamma-glutamic acid)-functionalized gold nanoparticles. Colloid Surface A 448:147–153
Ou KL, Hsu TC, Liu YC, Yang KH (2013) Controllably catalytic decomposition of acetaldehyde in solution by using gold nanoparticles released from sonoelectrochemically prepared gold microsheets. J Electroanal Chem 701:25–31
He H, Du J, Hu Y, Ru J, Lu X (2013) Detection of glutathione based on nickel hexacyanoferrate film modified Pt ultramicroelectrode by introducing cetyltrimethylammonium bromide and Au nanoparticles. Talanta 115:381–385
Shu HH, Chang G, Su J, Cao LL, Huang QW, Zhang YT, Xia TT, He YB (2015) Single-step electrochemical deposition of high performance Au-graphene nanocomposites for nonenzymatic glucose sensing. Sensor Actuat B-Chem 220:331–339
El-Deab MS, Okajima T, Ohsaka T (2003) Electrochemical reduction of oxygen on gold nanoparticle-electrodeposited glassy carbon electrodes. J Electrochem Soc 150(7):A851
Bard AJ, Faulkner LR (2001) Electrochemical methods: fundamentals and applications, 2nd edn. Wiley, New York
Ma H, Sun J, Zhang Y, Bian C, Xia S, Zhen T (2016) Label-free immunosensor based on one-step electrodeposition of chitosan-gold nanoparticles biocompatible film on Au microelectrode for determination of aflatoxin B1 in maize. Biosens Bioelectron 80:222–229
Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem 101(1):19–28
Chen Y, Gai PP, Zhang JR, Zhu JJ (2015) Design of an enzymatic biofuel cell with large power output. J Mater Chem A 3(21):11511–11516
Qu FL, Zhang Y, Rasooly A, Yang MH (2014) Electrochemical biosensing platform using hydrogel prepared from ferrocene modified amino acid as highly efficient immobilization matrix. Anal Chem 86(2):973–976
Campbell AS, Murata H, Carmali S, Matyjaszewski K, Islam MF, Russell AJ (2016) Polymer-based protein engineering grown ferrocene-containing redox polymers improve current generation in an enzymatic biofuel cell. Biosens Bioelectron 86(15):446–453
Kumar R, Leech D (2015) A glucose anode for enzymatic fuel cells optimized for current production under physiological conditions using a design of experiment approach. Bioelectrochemistry 106:41–46
Acknowledgments
This research is supported by the National Natural Science Foundation of China (Grant 51372206).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qu, F., Ma, X., Hui, Y. et al. Surfactant-assisted preparation of nanohybrid for simultaneously improving enzyme-immobilization and electron-transfer in biosensor and biofuel cell. J Solid State Electrochem 21, 1545–1557 (2017). https://doi.org/10.1007/s10008-017-3509-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-017-3509-3