Abstract
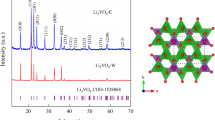
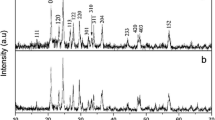
Here, we demonstrate a new, rapid, and flexible hydrothermal method using the V2O5 and LiOH as the precursors to synthesize Li3VO4. The ratios of precursor of V2O5 and LiOH can be changed in a wide range to control different preferred facets and morphologies, and the reason has been discussed from the structure of Li3VO4. The electrical performance of the Li3VO4 has also been systematically investigated. The thus-synthesized Li3VO4 exhibits significantly improved rate capability and cycling life compared with commercial graphite, synthesized Li4Ti5O12, and previously reported results on Li3VO4.





Similar content being viewed by others
References
Lin B, Yin Q, Hu H, Lu F, Xia H (2014) LiMn2O4 nanoparticles anchored on graphene nanosheets as high-performance cathode material for lithium-ion batteries. J Solid State Chem 209:23–28
Nair JR, Destro M, Bella F, Appetecchi GB, Gerbaldi C (2016) Thermally cured semi-interpenetrating electrolyte networks (s-IPN) for safe and aging-resistant secondary lithium polymer batteries. J Power Sources 306:258–267
Porcarelli L, Gerbaldi C, Bella F, Jijeesh RN (2016) Super soft all-ethylene oxide polymer electrolyte for safe all-solid lithium batteries. Sci Rep 6:19892 1-14
Yue L, Ma J, Zhang J, Zhao J, Dong S, Liu Z, Cui G (2016) All solid-state polymer electrolytes for high-performance lithium ion batteries. Energy Storage Materials 5:139–164
Cui L, Yang Y, Hsu C, Cui Y (2009) Carbon-silicon core-shell nanowires as high capacity electrode for lithium ion batteries. Nano Lett 9:3370–3374
Bae J (2011) Fabrication of carbon microcapsules containing silicon nanoparticles–carbon nanotubes nanocomposite by sol-gel method for anode in lithium ion battery. J Solid State Chem 184:1749–1755
Bruce P, Scrosati B, Tarascon J (2008) Nanomaterials for rechargeable lithium batteries. Angew Chem Int Ed 47:2930–2946
Wang X, Li M, Chang Z, Yang Y, Wu Y, Liu X (2015) Co3O4@MWCNT nanocable as cathode with superior electrochemical performance for supercapacitors. ACS Appl Mater Interf 7:2280–2285
Tang W, Liu L, Tian S, Li L, Yue Y, Wu Y, Zhu K (2011) Aqueous supercapacitors of high energy density based on MoO3 nanoplates as anode material. Chem Commun 47:10058–10060
Li H, Liu X, Zhai T, Li D, Zhou H (2012) Li3VO4: a promising insertion anode material for lithium-ion batteries. Adv Energy Mater 3:428–432
Placke T, Siozios V, Schmitz R, Lux SF, Bieker P, Colle C, Meyer HW, Passerini S, Winter M (2012) Influence of graphite surface modifications on the ratio of basal plane to “non-basal plane” surface area and on the anode performance in lithium ion batteries. J Power Sources 200:83–91
Armstrong A, Lyness C, Panchmatia P, Islam M, Bruce P (2011) The lithium intercalation process in the low-voltage lithium battery anode Li1+ xV1− xO2. Nat Mater 10:223–229
Malini R, Uma U, Sheela T, Ganesan M, Renganathan N (2009) Conversion reactions: a new pathway to realise energy in lithium-ion battery-review. Ionics 15:301–307
Liu L, Tang W, Tian S, Shi Y, Wu Y (2011) LiV3O8 nanomaterial as anode with good cycling performance for aqueous rechargeable lithium batteries. Funct Mater Lett 4:315–318
Tang W, Hou Y, Wang F, Liu L, Wu Y, Zhu K (2013) LiMn2O4 nanotube as cathode material of second-level charge capability for aqueous rechargeable batteries. Nano Lett 13:2036–2040
Cui L, Ruffo R, Chan CK, Peng H, Cui Y (2008) Crystalline-amorphous core- shell silicon nanowires for high capacity and high current battery electrodes. Nano Lett 9:491–495
Kim H, Bak S, Kim K (2010) Li4Ti5O12/reduced graphite oxide nano-hybrid material for high rate lithium-ion batteries. Electrochem Commun 12:1768–1771
Ohzuku T, Ueda A, Yamamoto N (1995) Zero-strain insertion material of Li[Li1/3Ti5/3]O4 for rechargeable lithium cells. J Electrochem Soc 142:1431–1435
Chen J, Yang L, Fang S, Hirano SI, Tachibana K (2012) Synthesis of hierarchical mesoporous nest-like Li4Ti5O12 for high-rate lithium ion batteries. J Power Sources 200:59–66
Song J, Park H, Kim K, Jo Y, Kim J, Jeong Y, Kim Y (2010) Electrochemical characteristics of lithium vanadate, Li1+ xVO2, new anode materials for lithium ion batteries. J Power Sources 195:6157–6161
Kim W, Jeong Y, Choi H, Kim Y, Song J, Lee H, Lee Y (2011) New anode materials of Li1+ xV1− xO2 (0≤ x≤ 0.1) for secondary lithium batteries: correlation between structures and properties. J Appl Electrochem 41:803–808
Shi Y, Wang J, Chou S, Wexler D, Li H, Ozawa K, Liu H, Wu Y (2013) Hollow structured Li3VO4 wrapped with graphene nanosheets in situ prepared by a one-pot template-free method as an anode for lithium-ion batteries. Nano Lett 13:4715–4720
Kim W, Jeong Y, Lee Y, Kim Y, Song J (2013) Synthesis and lithium intercalation properties of Li3VO4 as a new anode material for secondary lithium batteries. J Power Sources 244:557–560
Ke F, Huang L, Wei G, Xue L, Li J, Zhang B, Chen S, Fan X, Sun S (2009) One-step fabrication of CuO nanoribbons array electrode and its excellent lithium storage performance. Electrochim Acta 54:5825–5829
Kang B, Ceder G (2009) Battery materials for ultrafast charging and discharging. Nature 458:190–193
Wei GZ, Lu X, Ke FS, Huang L, Li JT, Wang ZX, Zhou ZY, Sun SG (2010) Crystal habit-tuned nanoplate material of Li[Li1/3–2×/3NixMn2/3–x/3]O2 for high-rate performance lithium-ion batteries. Adv Mater 22:4364–4367
Shi Y, Gao J, Abruña H, Li H, Liu H, Wexler D, Wang J, Wu Y (2014) The mechanism of the one-step synthesis of hollow-structured Li3VO4 as an anode for lithium-ion batteries. Chem-Eur J 20:5608–5612
Acknowledgements
Financial support from an Australian Research Council (ARC) Discovery Project (DP100103909), China National Distinguished Youth Scientists (NSFC No. 51425301) and STCSM (14520721800), is greatly appreciated. The authors acknowledge the use of facilities at the UOW Electron Microscopy Centre, including equipment funded by ARC Grant LE0237478. Many thanks also go to Dr. Tania Silver for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yi Shi and Yi Zhang have equal contribution to this work.
Rights and permissions
About this article
Cite this article
Shi, Y., Zhang, Y., Liu, L. et al. Rapid hydrothermal synthesis of Li3VO4 with different favored facets. J Solid State Electrochem 21, 2547–2553 (2017). https://doi.org/10.1007/s10008-016-3462-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-016-3462-6