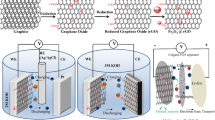
Abstract
Graphene nanosheets (G) and pure, as well as doped Mg-, Mn-, V-Li4Ti5O12, spinel structure have been synthesized. As-prepared materials were characterized by X-ray powder diffraction (XRD), FT-IR, scanning electron microscopy (SEM), cyclic voltammetry, and constant current discharge methods. The physical properties, as well as the possible role of the doped materials in supercapacitors, have been studied. The hybrid supercapacitor with pure or doped Li4Ti5O12 (LTO) anode was fabricated afterward to form the graphene/Li4Ti5O12. The specific energy, specific power, fast-charge capability, lifecycle, and self-discharge of the studied devices were compared. Metal doping did not change the phase structure while remarkably improved its capacitance at high charge/discharge rate. The hybrid supercapacitor utilizing pure or doped Li4Ti5O12 as an anode exhibits high capacitance compared to DLC because of the electrochemical process with intercalation/deintercalation of lithium into the spinel LTO. The capacitance of the hybrid supercapacitor decreases from 207 to 108 Fg−1 when discharged at several specific current densities ranging from 1 to 10 Ag−1. In contrast, the capacitance of the DLC is slightly decreased.










Similar content being viewed by others
References
Harrop DP, Zhitomirsky, DV (2013) Electrochemical DLC supercapacitors 2013–2023, business report IDTechEx, July 2013. Available at www.idtechex.com. Accessed March, 2015
Wang G, Zhang L, Zhang J (2012) A review of electrode materials for electrochemical supercapacitors. J Chem Soc Rev 41:797–828
Long JW, Bélanger D, Brousse T, Sugimoto W, Sassin MB, Crosnier O (2011) Asymmetric electrochemical capacitors-stretching the limits of aqueous electrolytes. J MRS Bull 36:513–522
Simon P, Gogotsi Y, Dunn B (2014) Materials science. Where do batteries end and supercapacitors begin?. Science 343:1210–1211
Jo MR, Lee GH, Kang YM (2015) Controlling solid- electrolyte- Interphase layer by coating P-type semiconductor NiOx on Li4Ti5O12 for high-energy-density lithium-ion batteries. J ACS Appl Mater Interfaces 7:27934–27939
Thackeray MM (1995) Structural considerations of layered and spinel lithiated oxides for lithium ion batteries. J Electrochem Soc 142:2558–2563
Jansen AN, Kahaian AJ, Kepler KD, Nelson PA, Amine K, Dees DW, Vissers DR (1999) Development of a high-power lithium-ion battery. J Power Sources 81-82:902–905
Goripart S, Miele E, Angelis FD, Fabrizio ED, Zaccaria RP, Capiglia C (2014) Review on recent progress of nanostructured anode materials for Li-ion batteries. J Power Sources 257:421–443
Baohua L, Feng N, Yan-Bing H, Hongda D, Quan-Hong Y, Jun M, Feiyu K, Chin-Tsau H (2011) Synthesis and characterization of long life Li4Ti5O12/C composite using amorphous TiO2 nanoparticles. Int J Electrochem Sci 6:3210–3223
Yi TF, Shu J, Zhu YR, Zhu XD, Zhu RS, Zhou AN (2010) Advanced electrochemical performance of Li4Ti4.95V0.05O12 as a reversible anode material down to 0V. J Power Sources 195:285–288
Huang S, Woodson M, Smalley R, Liu J (2004) Growth mechanism of oriented long single walled carbon nanotubes using “fast-heating” chemical vapor deposition process. J Nano Lett 4:1025–1028
Jung HG, Jang MW, Hassoun J, Sun YK, Scrosati B (2011) A high-rate long-life Li4Ti5O12/Li[Ni0.45Co0.1Mn1.45]O4 lithium-ion battery. J Nature Communications 2:516. doi:10.1038//ncomms1527
Li J, Tang J, Zhang Z (2005) Controllable formation and electrochemical properties of one-dimensional nanostructured spinel Li4Ti5O12. J Electrochem Commun 7:894–899
Kavana L, Grätzel M (2002) Facile synthesis of nanocrystalline Li4Ti5O12 (spinel) exhibiting fast Li insertion. J Electrochem Solid-State Lett 5:A39–A41
Yuan T, Cai R, Wang K, Ran R, Liu S, Shao Z (2009) Combustion synthesis of high-performance Li4Ti5O12 for secondary Li-ion battery. J Ceram Inter 35:1757–1768
Yao W, Zhuang W, Wang XJ (2016) Solid state synthesis of Li4Ti5O12 hiskers from TiO2-B. J Mater Res Bull 75:204–210
Birrozzi A, Copley M, Zamory J, Pasqualini M, Calcaterra S, Nobili F, Cicco AD, Rajantie H, Briceno M, Bilbé E, Cabo-Fernandez L, Hardwick LJ, Bresser D, Passerini S (2015) Scaling up nano Li4Ti5O12 for high-power lithium- ion anodes using large scale flame spray pyrolysis. J Electrochem Soc 162:A2331–A2338
Nowack LV, Waser O, Yarema O, Wood V (2013) Rapid, Microwave-assisted synthesis of battery-grade lithium titanate (LTO). J RSC Adv 3:15618–15621
Rao CNR, Sood AK (2013) Graphene: synthesis, properties, and phenomena. Wiley-VCH Verlag GmbH & Co. KgaA. doi:10.1002/9783527651122.ch1
Sheshmani S, Fashapoyeh MA (2013) Suitable chemical methods for preparation of graphene oxide, graphene and surface functionalized graphene nanosheets. J Acta Chim Slov 60:813–825
Klug HP, Alexander LE (1970) X-ray diffraction procedures. Wiley, New York
Laumann A (2010) Novel Routes to Li4Ti5O12 spinel: chatacterization and phase relations, Thesis, Facultät für Geowissenshaften der Ludwig Maximilians, Munchen University, Germany, 1
Sun X, Radovanovicb PV, Cuia B (2015) Advances in spinel Li4Ti5O12 anode materials for lithium-ion batteries. J New J Chem 39:38–63
Mingjia Z, Chengcheng X, Jiangtian L, Ming L, Nianqiang W (2013) Nanostructured carbon–metal oxide composite electrodes for supercapacitors: a review. Nanoscale 5:72
Gregg SJ, Sing KSW (1967) The adsorption, surface area and porosity. Academic, London
Woo SW, Dokko K, Kanamura K (2007) Preparation and characterization of three dimensionally ordered macroporous Li4Ti5O12 anode for lithium batteries. Electrochim Acta 53:79–82
Han D, Xu P, Jing X, Wang J, Yang P, Shen Q, Liu J, Song D, Gao Z, Zhang M (2013) Trisodium citrate assisted synthesis of hierarchical NiO nanospheres with improved supercapacitor performance. J Power Sources 235:45–53
Simon P, Gogotsi Y (2008) Materials for electrochemical capacitors. J Nature Mater 7:845–854
Reddy L, Endo T, Reddy GS (2012) Electronic (absorption) spectra of 3d transition metal complexes, chapter 1, advanced aspects of spectroscopy. Publisher: InTech
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khairy, M., Faisal, K. & Mousa, M. High-performance hybrid supercapacitor based on pure and doped Li4Ti5O12 and graphene. J Solid State Electrochem 21, 873–882 (2017). https://doi.org/10.1007/s10008-016-3433-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-016-3433-y