Abstract
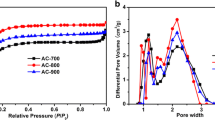
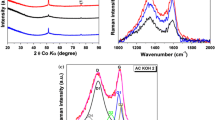
Activated carbon from tree bark (ACB) has been synthesized by a facile and environmentally friendly activation and carbonization process at different temperatures (600, 700 and 800 °C) using potassium hydroxide (KOH) pellets as an activation agent with different mass loading. The physicochemical and microstructural characteristics of the as-obtained material revealed interconnected microporous/mesoporous architecture with increasing trend in specific surface area (SSA) as carbonization temperatures rises. The SSA values of up to 1018 m2 g−1 and a high pore volume of 0.67 cm3 g−1 were obtained. The potential of the ACB material as suitable supercapacitor electrode was investigated in both a three and two-electrode configuration in different neutral aqueous electrolytes. The electrodes exhibited electric double-layer capacitor (EDLC) behaviour in all electrolytes with the Na2SO4 electrolyte working reversibly in both the negative (−0.80 V to −0.20 V) and positive (0.0 V to 0.6 V) operating potentials. A specific capacitance (C
s
) of up to 191 F g−1 at a current density of 1 A g−1 was obtained for the optimized ACB electrode material in 1 M Na2SO4 electrolyte. A symmetric device fabricated exhibited specific C
s
of 114 F g−1 at 0.3 A g−1 and excellent stability with a coulombic efficiency of a 100 % after 5000 constant charge–discharge cycles at 5.0 A g−1 and a low capacitance loss for a floating time of 70 h.

ᅟ









Similar content being viewed by others
References
Sun Y, Wu Q, Shi G (2011) Graphene based new energy materials. Energy Environ Sci 4:1113
Ghoniem AF (2011) Needs, resources and climate change: clean and efficient conversion technologies. Prog Energy Combust Sci 37:15–51
Patel MR (2005) Wind and solar power systems: design, analysis, and operation. CRC Press
Miller JR, Outlaw RA, Holloway BC (2010) Graphene double-layer capacitor with ac line-filtering performance. Science 329:1637–1639
Chmiola J, Largeot C, Taberna PL, Simon P, Gogotsi Y (2010) Monolithic carbide-derived carbon films for micro-supercapacitors. Science 328:480–483
Wei L, Sevilla M, Fuertes AB, Mokaya R, Yushin G (2012) Polypyrrole-derived activated carbons for high-performance electrical double-layer capacitors with ionic liquid electrolyte. Adv Funct Mater 22:827–834
Daffos B, Taberna PL, Gogotsi Y, Simon P (2010) Recent advances in understanding the capacitive storage in microporous carbons. Fuel Cells 10:819–824
Simon P, Gogotsi Y (2013) Capacitive energy storage in nanostructured carbon-electrolyte systems. Acc Chem Res 46:1094–1103
Miller JR, Burke AF (2008) Electrochemical capacitors: challenges and opportunities for real-world applications. Electrochem Soc 17:53–57
Béguin F, Presser V, Balducci A, Frackowiak E (2014) Carbons and electrolytes for advanced supercapacitors. Adv Mater 26(2219–51):2283
Conway BE, Birss V, Wojtowicz J (1997) The role and utilization of pseudocapacitance for energy storage by supercapacitors. J Power Sources 66:1–14
Wei L, Yushin G (2012) Nanostructured activated carbons from natural precursors for electrical double layer capacitors. Nano Energy 1:552–565
Simon P, Gogotsi Y (2008) Materials for electrochemical capacitors. Nat Mater 7:845–854
Lillo-Ródenas MA, Cazorla-Amorós D, Linares-Solano A (2005) KOH and NaOH activation mechanisms of multiwalled carbon nanotubes with different structural organisation. Carbon 43:786–795
Chowdhury ZZ, Hamid SBA, Das R, Hasan MR, Zain SM, Khalid K, Uddin MN (2013) Preparation of carbonaceous adsorbents from lignocellulosic biomass and their use in removal of contaminants from aqueous solution. Bioresources 8:6523–6555
Lillo-Ródenas MA, Cazorla-Amorós D, Linares-Solano A (2003) Understanding chemical reactions between carbons and NaOH and KOH: an insight into the chemical activation mechanism. Carbon 41:267–275
Yang K, Peng J, Srinivasakannan C, Zhang H, Xia H, Duan X (2010) Preparation of high surface area activated carbon from coconut shells using microwave heating. Bioresour Technol 101:6163–6169
Zhi M, Yang F, Meng F, Li M, Manivannan A, Wu NN (2014) Effects of pore structure on performance of an activated-carbon supercapacitor electrode recycled from scrap waste tire effects of pore structure on performance of an activated-carbon supercapacitor electrode recycled from scrap waste tire. ACS Sustain Chem Eng 2:1592–1598
Wei L, Yushin G (2011) Electrical double layer capacitors with activated sucrose-derived carbon electrodes. Carbon 49:4830–4838
Stavropoulos GG, Zabaniotou AA (2009) Minimizing activated carbons production cost. Fuel Process Technol 90:952–957
Divyashree A, Gurumurthy H (2015) Activated carbon nanospheres derived from bio- waste materials for supercapacitor applications—a review. RSC Adv 5:88339–88352
Abbas Q, Pajak D, Frackowiak E, Beguin F (2014) Effect of binder on the performance of carbon/carbon symmetric capacitors in salt aqueous electrolyte. Electrochim Acta 140:132–138
Hong MS, Lee SH, Kim SW (2002) Use of KCl aqueous electrolyte for 2 V manganese oxide/activated carbon hybrid capacitor. Electrochem Solid-State Lett 5:A227
Bichat MP, Raymundo-Piñero E, Béguin F (2010) High voltage supercapacitor built with seaweed carbons in neutral aqueous electrolyte. Carbon 48:4351–4361
Zhang H, Zhang L, Chen J, Su H, Liu F, Yang W (2016) One-step synthesis of hierarchically porous carbons for high-performance electric double layer supercapacitors. J Power Sources 315:120–126
Wang JG, Yang Y, Huang ZH, Kang F (2013) A high-performance asymmetric supercapacitor based on carbon and carbon–MnO2 nanofiber electrodes. Carbon 61:190–199
Dyatkin B, Presser V, Heon M, Lukatskaya MR, Beidaghi M, Gogotsi Y (2013) Development of a green supercapacitor composed entirely of environmentally friendly materials. ChemSusChem 6:2269–2280
Veeramani V, Madhu R, Chen SM, Veerakumar P, Syu JJ, Liu SB (2015) Cajeput tree bark derived activated carbon for the practical electrochemical detection of vanillin. New J Chem 39:9109–9115
Koutcheiko S, Vorontsov V (2013) Activated carbon derived from wood biochar and its application in supercapacitors. J Biobased Mater Bioenergy 7:733–740
Wang J, Song Y, Li Z, Liu Q, Zhou J, Jing X, Zhang M, Jiang Z (2010) In situ Ni/Al layered double hydroxide and its electrochemical capacitance performance. Energy Fuel 24:6463–6467
Yang W, Gao Z, Wang J, Ma J, Zhang M, Liu L (2013) Solvothermal one-step synthesis of Ni-Al layered double hydroxide/carbon nanotube/reduced graphene oxide sheet ternary nanocomposite with ultrahigh capacitance for supercapacitors. ACS Appl Mater Interfaces 5:5443–5454
Barzegar F, Bello A, Fashedemi OO, Dangbegnon JK, Momodu DY, Taghizadeh F, Manyala N (2015) Synthesis of 3D porous carbon based on cheap polymers and graphene foam for high-performance electrochemical capacitors. Electrochim Acta 180:442–450
Sun H, He W, Zong C, Lu L (2013) Template-free synthesis of renewable macroporous carbon via yeast cells for high-performance supercapacitor electrode materials. ACS Appl Mater Interfaces 5:2261–2268
Wei L, Tian K, Jin Y, Zhang X, Guo X (2016) Three-dimensional porous hollow microspheres of activated carbon for high-performance electrical double-layer capacitors. Microporous Mesoporous Mater 227:210–218
Li H, Kang Z, Liu Y, Lee ST (2012) Carbon nanodots: synthesis, properties and applications. J Mater Chem 22:24230
Bello A, Manyala N, Barzegar F, Khaleed AA, Momodu DY, Dangbegnon JK (2016) Renewable pine cone biomass derived carbon materials for supercapacitor application. RSC Adv 6:1800–1809
Luo QP, Huang L, Gao X, Cheng Y, Yao B, Hu Z, Wan J, Xiao X, Zhou J (2015) Activated carbon derived from melaleuca barks for outstanding high-rate supercapacitors. Nanotechnology 26:304004
Mao Y, Duan H, Xu B, Zhang L, Hu Y, Zhao C, Wang Z, Chen L, Yang Y (2012) Lithium storage in nitrogen-rich mesoporous carbon materials. Energy Environ Sci 5:7950
Sadezky A, Muckenhuber H, Grothe H, Niessner R, Poschl U (2005) Raman microspectroscopy of soot and related carbonaceous materials: spectral analysis and structural information. Carbon 43:1731–1742
Malard LMM, Pimenta MAA, Dresselhaus G, Dresselhaus MSS (2009) Raman spectroscopy in graphene. Phys Rep 473:51–87
Wang Y, Alsmeyer DC, McCreery RL (1990) Raman spectroscopy of carbon materials: structural basis of observed spectra. Chem Mater 2:557–563
Jawhari T, Roid A, Casado J (1995) Raman spectroscopic characterization of some commercially available carbon black materials. Carbon 33:1561–1565
Sze SK, Siddique N, Sloan JJ, Escribano R (2001) Raman spectroscopic characterization of carbonaceous aerosols. Atmos Environ 35:561–568
Dippel B, Jander H, Heintzenberg J (1999) NIR FT Raman spectroscopic study of flame soot. Phys Chem Chem Phys 1:4707–4712
Yao L, Yang G, Han P, Tang Z, Yang J (2016) Three-dimensional beehive-like hierarchical porous polyacrylonitrile-based carbons as a high performance supercapacitor electrodes. J Power Sources 315:209–217
Ofomaja AE, Naidoo EB (2011) Biosorption of copper from aqueous solution by chemically activated pine cone: a kinetic study. Chem Eng J 175:260–270
Xie K, Qin X, Wang X, Wang Y, Tao H, Wu Q, Yang L, Hu Z (2012) Carbon nanocages as supercapacitor electrode materials. Adv Mater 24:347–352
Barzegar F, Momodu DY, Fashedemi OO, Bello A, Dangbegnon JK, Manyala N (2015) Investigation of different aqueous electrolytes on the electrochemical performance of activated carbon-based supercapacitors. RSC Adv 5:107482–107487
Toupin M, Bélanger D, Hill IR, Quinn D (2005) Performance of experimental carbon blacks in aqueous supercapacitors. J Power Sources 140:203–210
Zhong C, Deng Y, Hu W, Qiao J, Zhang L, Zhang J (2015) A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem Soc Rev 44:7484–7539
Zhang C, Hatzell KB, Boota M, Dyatkin B, Beidaghi M, Lng D, Qiao W, Kumbur EC, Gogotsi Y (2014) Highly porous carbon spheres for electrochemical capacitors and capacitive flowable suspension electrodes. Carbon 77:155–164
Fan Z, Yan J, Wei T, Zhi L, Ning G, Li T, Wei F (2011) Asymmetric supercapacitors based on graphene/MnO2 and activated carbon nanofiber electrodes with high power and energy density. Adv Funct Mater 21:2366–2375
Randles JEB (1947) Kinetics of rapid electrode reactions. Discuss Faraday Soc 1:11–19
Conway B (1999) Electrochemical supercapacitors: scientific fundamentals and technological applications. Kluwer Academic Publishers, Plenum Press, New York
Bohlen O, Kowal J, Sauer DU (2007) Ageing behaviour of electrochemical double layer capacitors. Part I. Experimental study and ageing model. J Power Sources 172:468–475
Bohlen O, Kowal J, Sauer DU (2007) Ageing behaviour of electrochemical double layer capacitors. Part II. Lifetime simulation model for dynamic applications. J Power Sources 173:626–632
Saha D, Li Y, Bi Z, Chen J, Keum JK, Hensley DK, Grappe HA, Meyer HM, Dai S, Paranthaman MP, Naskar AK (2014) Studies on supercapacitor electrode material from activated lignin- derived mesoporous carbon. Langmuir 30:900–910
Volperts A, Dobele G, Ozolins J, Mironova-Ulmane N (2015) Synthesis and application of nanoporous activated carbon in supercapacitors. Mater Sci Appl Chem 31:16
Ferrero GA, Fuertes AB, Sevilla M (2015) From soybean residue to advanced supercapacitors. Sci Rep 5:16618
Wu ZS, Ren W, Wang DW, Li F, Liu B, Cheng HM (2010) High-energy MnO2 nanowire/graphene and graphene asymmetric electrochemical capacitors. ACS Nano 4:5835–5842
Wu FC, Tseng RL, Hu CC, Wang CC (2005) Effects of pore structure and electrolyte on the capacitive characteristics of steam- and KOH-activated carbons for supercapacitors. J Power Sources 144:302–309
Syarif N, Tribidasari I, Wibowo W (2013) Binder-less activated carbon electrode from gelam wood for use in supercapacitors. J Electrochem Sci Eng 3:37–45
Ratajczak P, Jurewicz K, Béguin F (2014) Factors contributing to ageing of high voltage carbon/carbon supercapacitors in salt aqueous electrolyte. J Appl Electrochem 44:475–480
Zhang L, Wang J, Zhu J, Zhang X, San Hui K, Hui KN (2013) 3D porous layered double hydroxides grown on graphene as advanced electrochemical pseudocapacitor materials. J Mater Chem A 1:904
Acknowledgments
The authors would like to specially thank Isbe VanDerWesthuizen for the results obtained from the TGA analysis. This work is based on the research supported by the South African Research Chairs Initiative of the Department of Science and Technology, Republic of South Africa and National Research Foundation of South Africa (Grant no. 97994). Any opinion, finding and conclusion or recommendation expressed in this material is that of the author(s) and the NRF does not accept any liability in this regard. D. Momodu and F. Barzegar would like to acknowledge financial support from the University of Pretoria for their post-doctoral study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supporting Information 1
(DOCX 8429 kb)
Rights and permissions
About this article
Cite this article
Momodu, D., Madito, M., Barzegar, F. et al. Activated carbon derived from tree bark biomass with promising material properties for supercapacitors. J Solid State Electrochem 21, 859–872 (2017). https://doi.org/10.1007/s10008-016-3432-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-016-3432-z