Abstract
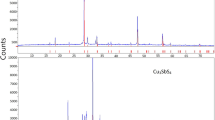
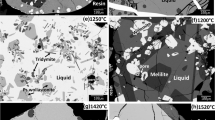
Thermodynamic properties of solid phases in the Cu–O–Al2O3 system were measured by means of the EMF method with oxygen concentration galvanic cells based on stabilized zirconia solid electrolytes. The standard Gibbs energies of formation of pure copper oxides and copper aluminates from their component oxides were determined. Copper aluminates were also investigated calorimetrically by the DSC-TGA combined techniques. Based on the calorimetric measurements, the enthalpies and temperatures of spinel decomposition and delafossite melting were determined. The obtained results were discussed and compared with the available literature data.













Similar content being viewed by others
References
Schlesinger ME, King MJ, Sole KC, Davenport WGI (2011) Extractive metallurgy of copper (chapter 18), fifth edn. Elsevier, Oxford
Malfliet A, Lotfian S, Scheunis L, Petkov V, Pandelaers L, Jones PT, Blanpain B (2014) Degradation mechanisms and use of refractory linings in copper production processes: a critical review. J Eur Ceram Soc 34(3):849–876
Rigby GR, Hamilton B (1961) A study of basic brick from copper anode furnaces. J Am Ceram Soc 44:201–205
Jacob KT, Alcock CB (1975) Thermodynamics of CuAlO2 and CuAl2O4 and phase equilibria in the system Cu2O-CuO-Al2O3. J Am Ceram Soc 58(5–6):192–195
Fujimura T, Tanaka S-I (1998) In-situ high temperature X-ray diffraction study of Cu/Al2O3 interface reactions. Acta Mater 46(9):3057–3061
YI S, Trumble KP, Gaskell DR (1999) Thermodynamic analysis of aluminate stability in the eutectic bonding of copper with alumina. Acta Mater 47(11):3221–3226
Seager CW, Kokini K, Trumble K, Krane MJM (2002) The influence of CuAlO2 on the strength of eutectically bonded Cu/Al2O3 interfaces. Scr Mater 46:395–400
Silvain JF, Bobet JL, Heintz JM (2002) Electroless deposition of copper onto alumina sub-micronic powders and sintering. Compos A: Appl Sci Manuf 33A(10):1387–1390
Tahir D, Tougaard S (2012) Electronic and optical properties of Cu, CuO and Cu2O studied by electron spectroscopy. J Phys Condens Matter 24(17):175002/1–175002/8
Robertson J, Peacock PW, Towler MD, Needs R (2002) Electronic structure of p-type conducting transparent oxides. Thin Solid Films 411(1):96–100
Arjmand M, Knee CS, Leion H, Mattisson T (2015) Standard enthalpy of formation of CuAl2O4 revisited. Chem Eng Commun 202(5):694–697
Hallstedt B, Risold D, Gauckler LJ (1994) Thermodynamic assessment of the copper-oxygen system. J Phase Equilib 15(5):483–499
Hallstedt B, Gauckler LJ (2003) Revision of the thermodynamic descriptions of the Cu-O, Ag-O, Ag-Cu-O, Bi-Sr-O, Bi-Ca-O, Bi-Cu-O, Sr-Cu-O, Ca-Cu-O and Sr-Ca-Cu-O systems. Calphad 27(2):177–191
Clavaguera-Mora MT, Touron JL, Rodriguez-Viejo J, Clavaguera N (2004) Thermodynamic description of the Cu-O system. J Alloys Compd 377(1–2):8–16
Shishin D, Decterov SA (2012) Critical assessment and thermodynamic modeling of the Cu-O and Cu-O-S systems. Calphad 38:59–70
Roberts HS, Smith FH (1921) The system copper: cupric oxide: oxygen. J Am Ceram Soc 43:1061–1070
Gadalla AMM, Ford WF, White J (1963) Equilibrium relations in the system CuO-Cu2O-SiO2. Brit Ceram Trans J 62(1):45–66
Fitzner K, Moser Z (1979) Activity of oxygen in dilute liquid copper-oxygen alloys. Metals Technol (London) 6(7):273–275
Boudene A, Hack K, Mohammad A, Neuschutz D, Zimmermann E (1992) Experimental investigation and thermochemical assessment of the system copper-oxygen. Zeitschrift fuer Metallkunde 83(9):663–668
Kosenko AV, Emel’chenko GA (2001) Equilibrium phase relationships in the system Cu-O under high oxygen pressure. J Phase Equilib 22(1):12–19
Schmalzried H (1960) Measurement of the free enthalpy of reaction in the formation of spinel phases from the single oxides by aid of solid galvanic couples. Zeitschrift fuer Physikalische Chemie (Muenchen, Germany) 25:178–192
Misra SK, Chaklader ACD (1963) The system copper oxide–alumina. J Am Ceram Soc 46(10):509
Gadalla AMM, White J (1964) Equilibrium relation in the system Cu2O-CuO-Al2O3. Brit Ceram Trans J 63(1):39–62
Navrotsky A, Kleppa OJ (1968) Thermodynamics of formation of simple spinels. J Inorg Nucl Chem 30(2):479–498
Zalazinskii AG, Balakirev VF, Chebotaev NM, Chufarov GI (1969) Thermodynamic analysis of the reduction, dissociation, and formation of copper aluminate, copper chromate(III), and copper ferrate(II) from the free elements and oxides. Zhurnal Neorganicheskoi Khimii 14(3):624–626
Slobodyanyuk AA, Tret’yakov YD, Bessonov AF (1971) Thermodynamic stability of copper silicates and aluminates studied by emf. with a solid electrolyte. Zhurnal Fizicheskoi Khimii 45(7):1871–1872
Tsuchida T, Furuichi R, Sukegawa T, Furudate M, Ishii T (1984) Thermoanalytical study on the reaction of the CuO-Al2O3 (η, γ and α) systems. Thermochim Acta 78(1–3):71–80
Trumble KP (1992) Thermodynamic analysis of aluminate formation at Fe/Al2O3 and Cu/Al2O3 interfaces. Acta Metall Mater 40(Suppl):S105–S110
Trumble KP (1999) Prediction of a critical temperature for aluminate formation in alumina/copper-oxygen eutectic bonding. J Am Ceram Soc 82(10):2919–2920
Guedes M, Ferreira JMF, Ferro AC (2008) A study on CuO-Al2O3 reaction paths. Adv Powder Metall Part Mater 5/1–5/13
Chen M, Zhao B (2013) Phase equilibrium studies of "Cu2O"-SiO2-Al2O3 system in equilibrium with metallic copper. J Am Ceram Soc 96(11):3631–3636
Hellstén N, Hamuyuni J, Taskinen P (2016) High-temperature phase equilibria of Cu-O-Al2O3 system in air. Can Metall Q 55(2):226–233
Åsbrink S, Waskowska A (1991) CuO: X-ray single-crystal structure determination at 196 K and room temperature. J Phys Condens Matter 3(42):8173–8180
Restori R, Schwarzenbach D (1986) Charge density in cuprite, Cu2O. Acta Crystallogr Sect B: Struct Sci B42(3):201–208
O’Neill HSC, James M, Dollase WA, Redfern SAT (2005) Temperature dependence of the cation distribution in CuAl2O4 spinel. Eur J Mineral 17(4):581–586
Ishiguro T, Kitazawa A, Mizutani N, Kato M (1981) Single-crystal growth and crystal structure refinement of CuAlO2. J Solid State Chem 40(2):170–174
Cohen ER, Cvitaš T, Frey JG, Holmström B, Kuchitsu K, Marquardt R, Mills I, Pavese F, Quack M, Stohner J, Strauss HL, Takami M, Thor AJ (2007) Quantities, units, and symbols in physical chemistry IUPAC green book, 3rd edn. IUPAC and RCS Publishing, Cambridge
Holmes RD, O’Neill HSC, Arculus RJ (1986) Standard Gibbs free energy of formation for Cu2O, NiO, CoO, and FexO: high resolution electrochemical measurements using zirconia solid electrolytes from 900 to 1400 K. Geochim Cosmochim Acta 50(11):2439–2452
Barin I (1989) Thermodynamical data of pure substances, part I, VCH Verlagsgesellschaft, Weinheim. VCH Publishers, New York
Acknowledgments
This work was financially supported by the Finnish Metals Producers Fund and the Academy of Finland.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PDF 264 kb)
Rights and permissions
About this article
Cite this article
Sukhomlinov, D., Tesfaye, F., Hellstén, N. et al. Thermodynamic stability of solid phases in the system Cu–O–Al2O3 by means of the EMF and DSC-TGA techniques. J Solid State Electrochem 22, 959–972 (2018). https://doi.org/10.1007/s10008-016-3430-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-016-3430-1