Abstract
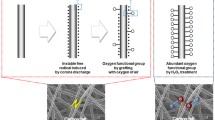
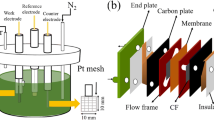
Electrodes for large-scale usage in vanadium redox flow battery are usually fabricated without any electrocatalyst due to the lack of good, viable options. The best performance is achieved of carbon-based materials. Recently, some researchers have been reported regarding the use of carbon nanotube as the electrocatalyst in the vanadium redox flow batteries. However, these researches have been carried out without making any comparison between the performance of the traditional method and the carbon nanotube electrocatalyst. In the present study, the loading of multi-walled carbon nanotube, the acid–heat treatment, and their combination were used to modify the carbon felt electrode to be applied in the vanadium redox flow battery. The obtained results showed better electrochemical properties for acid–heat-treated carbon felt electrode compared to the carbon nanotube-loaded one. The best electrode was obtained for using in a vanadium redox flow battery in terms of electrochemical and surface properties after applying a combination of two modification strategies. Applying this proposed method in modification of the carbon felt electrode increased its hydrophilicity more than 17 times and its capability to absorb VOSO4 solution more than eight times. Also, the charge transfer resistance of a modified electrode, by the combination of the carbon nanotube and the acid–heat treatment, significantly decreased in both positive and negative poles of vanadium redox flow battery. Consequently, the exchange current density enhanced more than 100- and 175-fold in positive and negative poles, respectively, in comparison with carbon felt electrode.







Similar content being viewed by others
References
Ginley D, Green MA, Collins R (2008) Solar energy conversion toward 1 terawatt. MRS Bull 33:355–364
Lee BS, Gushee DE (2008) Electricity storage: the Achilles heels of renewable energy. Chem Eng Progress 104:S29–S32
Pool R (2007) Climate change stirs storage industry. Power Eng J 21:10–11
Fabjan C, Garche J, Harrer B, Jörissen L, Kolbeck C, Philippi F, Tomazic G, Wagner F (2001) The vanadium redox-battery: an efficient storage unit for photovoltaic systems. Electrochim Acta 47:825–831
Gattrell M, Park J, MacDougall B, Apte J, McCarthy S, Wu CW (2003) Study of the mechanism of the vanadium 4+/5+ redox reaction in acidic solution. J Electrochem Soc 151:123–130
Li W, Liu J, Yan C (2011) Multi-walled carbon nanotubes used as an electrode reaction catalyst for VO2 +/VO2+ for a vanadium redox flow battery. Carbon 49:3463–3470
González Z, Botas C, Álvarez P, Roldán S, Blanco C, Santamaría R, Granda M, Menéndez R (2012) Thermally reduced graphite oxide as positive electrode in vanadium redox flow batteries. Carbon 50:828–834
Kear G, Shah AA, Walsh FC (2012) Development of the all-vanadium redox flow battery for energy storage: a review of technological, financial and policy aspects. Int J Energ Res 36:1105–1120
Kim HS (2011) Electrochemical properties of graphite-based electrodes for redox flow batteries. B Kor Chem Soc 32:571–575
Wang W, Wang X (2007) Investigation of Ir-modified carbon felt as the positive electrode of an all-vanadium redox flow battery. Electrochim Acta 52:6755–6762
Tsai HM, Yang SY, Ma CCM, Xie X (2011) Preparation and electrochemical properties of graphene-modified electrodes for all-vanadium redox flow batteries. Electroanal 23:2139–2143
González Z, Sanchez A, Blanco C, Granda M, Menéndez R, Santamaría R (2011) Enhanced performance of a Bi-modified graphite felt as the positive electrode of a vanadium redox flow battery. Electrochem Commun 13:1379–1382
Rychcik M, Skallas-Kazacos M (1987) Evaluation of electrode materials for vanadium redox cell. J Power Sources 19:45–54
Yao C, Zhang H, Liu T, Li X, Liu Z (2012) Carbon paper coated with supported tungsten trioxide as novel electrode for all-vanadium flow battery. J Power Sources 218:455–461
Tan CW, Tan KH, Ong YT, Mohamed AR, Zein SHS, Tan SH (2012) Energy and environmental applications of carbon nanotubes. Environ Chem Lett 10:265–273
Wei G, Jia C, Liu J, Yan C (2012) Carbon felt supported carbon nanotubes catalysts composite electrode for vanadium redox flow battery application. J Power Sources 220:185–192
Sun B, Skyllas-Kazakos M (1991) Chemical modification and electrochemical behavior of graphite fiber in acidic vanadium solution. Electrochim Acta 36:513–517
Sun B, Skyllas-Kazacos M (1992) Modification of graphite electrode materials for vanadium redox flow battery application—I. Thermal treatment. Electrochim Acta 37:1253–1260
Sun B, Skyllas-Kazacos M (1992) Chemical modification of graphite electrode materials for vanadium redox flow battery application—part II. Acid treatments. Electrochim Acta 37:2459–2465
Li W, Liu J, Yan C (2012) The electrochemical catalytic activity of single-walled carbon nanotubes towards VO2+/VO2 + and V3+/V2+ redox pairs for an all vanadium redox flow battery. Electrochim Acta 79:102–108
Mahajan A, Kingon A, Kukovecz A, Konya Z, Vilarinho PM (2013) Studies on the thermal decomposition of multiwall carbon nanotubes under different atmospheres. Mater Lett 90:165–168
Hsieh Y-C, Chou Y-C, Lin C-P, Hsieh T-F, Shu C-M (2010) Thermal analysis of multi-walled carbon nanotubes by Kissinger’s corrected kinetic equation. Aerosol Air Qual Res 10:212–218
Bard AJ, Faulkner LR (2001) Electrochemical methods fundamentals and application. Wiley, New York
Lefrou C, Fabry P, Poignet J-C (2012) Electrochemistry: the basics, with examples. Springer, Heidelberg
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jelyani, M.Z., Rashid-Nadimi, S. & Asghari, S. Treated carbon felt as electrode material in vanadium redox flow batteries: a study of the use of carbon nanotubes as electrocatalyst. J Solid State Electrochem 21, 69–79 (2017). https://doi.org/10.1007/s10008-016-3336-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-016-3336-y