Abstract
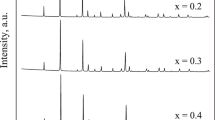
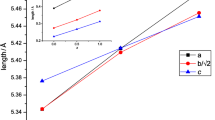
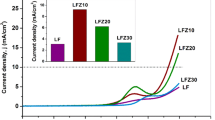
In this paper, the electrochemical behavior of europium perovskites (Ca0.6Eu0.4MnO3) prepared by a gel combustion method followed by a thermal treatment performed at 1073 and 1473 K is compared with the pristine oxide CaMnO3 (T = 1073 K) obtained by the same method. The experiments were performed in alkaline aqueous media via open-circuit potential, cyclic voltammetry, chronopotentiometry, and impedance spectroscopy. The data show that the electrode roughness is inversely proportional to the oxide particle size. The chronopotentiometric curves show also that the presence of europium is advantageous due to the increase of the electrode roughness for the oxides formed at the same temperature (1073 K) or by increasing the charge per unit area for the oxide formed at 1473 K. The impedance spectra, which were obtained in the capacitive behavior domain, reflect the porous morphology of the electrode surfaces according to the theory developed by Levie. This feature is particularly evident for the electrodes with an average particle diameter of 90 and 60 nm, corresponding respectively to the oxides CaMnO3 and Ca0.6Eu0.4MnO3, both formed at 1073 K.









Similar content being viewed by others
References
Ni C, Irvine JTS (2015) Calcium manganite as oxygen electrode materials for reversible solid oxide fuel cell. Faraday Discuss 182:289–305
Lay E, Benamira M, Pirovano C, Gauthier G, Dessemond L (2012) Effect of Ce-doping on the electrical and electrocatalytical behaviour of La/Sr chromo-manganite perovskite as new SOFC anode. Fuel Cells 12:265–274
Marina OA, Pederson LR, Williams MC, Coffey GW, Meinhardtn KD, Nguyen CD, Thomsen EC (2007) Electrode performance in reversible solid oxide fuel cells. J Electrochem Soc 154:B452–B459
Bernuy-Lopez C, Knibbe R, He Z, Mao X, Hauch A, Nielsen KA (2011) Electrochemical characterization of solid oxide cell electrodes for hydrogen production. J Power Sources 196:4396–4403
Morimoto H, Esaka T, Takai S (1997) Properties of the perovskite-type oxide ceramic Ca1-x La2x/3MnO3-δ as the cathode active materials in alkaline batteries. Mater Res Bull 32:1359–1366
Esaka T, Adachi Y (2014) Electrode property of sintered ceramic based on CaMnO3 in LiOH aqueous solution. J Mater Sci Chem Eng 2:15–21
Zhang K, Han X, Hu Z, Zhang X, Tao Z, Chen J (2015) Nanostructured Mn-based oxides for electrochemical energy storage and conversion. Chem Soc Rev 44:699–728
Han X, Hu Y, Yang J, Cheng F, Chen J (2014) Porous perovskite CaMnO3 as an electrocatalyst for rechargeable Li–O2 batteries. Chem Commun 50:1497–1499
Fu Z, Lin X, Huang T, Yu A (2012) Nano-Sized La0.8Sr0.2MnO3 as oxygen reduction catalyst in nonaqueous Li/O2 batteries. J Solid State Electrochem 16:1497–1452
Li G, Zhang K, Mezaal MA, Lei L (2015) Synthesis and electrochemical evaluation of La1−x Sr x MnO3 catalysts for zinc-air batteries. J Solid State Electrochem. doi:10.1007/s10008-015-3056-8
Li G, Mezzal MA, Zhang R, Zhang K, Liu W, Lei L (2015) Electrochemical evaluation of La1−x Sr x MnO3 in zinc-air batteries. Int J Electrochem Sci 10:8412–8422
Du J, Zhang T, Cheng F, Chu W, Wu Z, Chen J (2014) Nonstoichiometric perovskite CaMnO3−δ for oxygen electrocatalysis with high activity. J Inorg Chem 53:9106–9114
Raabe S, Meirwaldt D, Ciston J, Uijttewaal M, Stein H, Hoffman J, Zhu Y, Blöchl P, Jooss C (2012) In situ electrochemical electron microscopy study of oxygen evolution activity of doped manganite perovskites. Adv Funct Mater 22:3378–3388
Suntivivh J, May KJ, Gasteiger HA, Goodenough JB, Shao-Horn Y (2011) A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 334:1383–1385
Mierwaldt D, Mildner S, Arrigo R, Knop-Gericke A, Franke E, Blumenstein A, Hoffmann J, Jooss C (2014) In situ XANES/XPS investigation of doped manganese perovskite catalysts. Catal 4:129–145
Han X, Cheng F, Zhang T, Jingang Y, Hu Y, Chen J (2014) Hydrogenated uniform Pt clusters supported on porous CaMnO3 as a bifunctional electrocatalyst for enhanced oxygen reduction and evolution. Adv Mater 26:2047–2051
Peña MA, Fierro JLG (2001) Chemical structures and performance of perovskite oxides. Chem Rev 101:1981–2017
Melo Jorge ME, Nunes MR, Silva Maria R, Sousa D (2005) Insulator-metal transition induced by Ce doping in CaMnO3. Chem Mater 17:2069–2075
Isasi PH, Lopes ME, Nunes MR, Melo Jorge ME (2009) Low-temperature synthesis of nanocrystalline Ca1-x Ho x MnO3-δ (0 < x < 0.3) powders. J Phys Chem Solids 70:405–411
Matos I, Sério S, Lopes ME, Nunes MR, Melo Jorge ME (2011) Effect of the sintering temperature on the properties of nanocrystalline Ca1-x Sm x MnO3 (0 ≤ x ≤ 0.4) powders. J Alloys Compds 509:9617–9626
Silveira C, Lopes ME, Nunes MR, Melo Jorge ME (2010) Synthesis and electrical properties of nanocrystalline Ca1−x Eu x MnO3±δ (0.1 ≤ x ≤ 0.4) powders prepared at low temperature using citrate gel method. Solid State Ionics 180:1702–1709
Sousa D, Nunes MR, Lopes AB, Melo Jorge ME (2008) Ca-site doping induced a metal-insulator transition in manganite CaMnO3. Mater Chem Phys 109:311–319
Najjar H, Batis H, Lamonier JF, Mentréa O, Giraudon JM (2014) Effect of praseodymium and europium doping in La1−x LnxMnO3+ı (ln: Pr or Eu, 0 ≤ x ≤ 1) perosvkite catalysts for total methane oxidation. Appl Catal A Gen 469:98–107
Phuruangrat A, Yayapao O, Thongtem T, Thongtem S (2014) Synthesis and characterization of europium-doped zinc oxide photocatalyst. J Nanomaterials 2014:(Article ID 367529), 9 pages
Zhao Y, Zhang C, Liu T, Du ZJ, Liang S, Wang X (2012) Electrocatalytic activity of europium doped tantalum oxide for ascorbic acid oxidation in aqueous. Int J Electrochem Sci 7:6417–6425
Moustafa MSA (ed) (2013) Europium: synthesis, characteristics and potential applications. Nova Science publishers, New York
Melo Jorge ME, Correia dos Santos A, Nunes MR (2001) Effects of synthesis method on stoichiometry, structure and electrical conductivity of CaMnO3-δ. Int J Inorg Mater 3:915–921
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr Sect A: Found Crystallogr 32:751–767
Pourbaix M (1974) Atlas of electrochemical equilibria in aqueous solutions. NACE, Houston
Ferreira BM, Melo Jorge ME, Lopes ME, Nunes MR, Silva Pereira MI (2009) Properties of Ca1-x Ho x MnO3 perovskite-tipe electrodes. Electrochim Acta 54:5902–5908
Barrocas B, Sério S, Rovisco A, Nunes Y, Sá AI, Silva Pereira MI, Melo Jorge ME (2014) Characterization and electrochemical behaviour of nanostructured calcium samarium manganite electrodes fabricated by RF-magnetron sputtering. Electrochim Acta 137:99–107
Lucas C, Eiroa I, Nunes MR, Russo PA, Ribeiro Carrott MML, Silva Pereira MI, Melo Jorge ME (2009) Preparation and characterization of Ca1-x Ce x MnO3 perovskite electrodes. J Solid State Electrochem 13:943–950
Bockris LOM, Sum K (1993) Surface electrochemistry, a molecular level approach. Plenum, New York
Silva LM, Faria LA, Boodts JFC (2001) Determination of the morphology factor of oxide layers. Electrochim Acta 47:395–403
Levine S, Smith AL (1971) Theory of the differential capacity of the oxide/aqueous electrolyte interface. Discuss Faraday Soc 52:290–301
Greef R, Peat R, Peter LM, Pletcher D, Ronbison J (1985) Instrumental methods in electrochemistry. Ellis Horwood Lmd, Chichester
Brett AMO, Brett CMA (1993) Electrochemistry: principles, methods and applications. Oxford University Press, Oxford
Levie R (1963) On porous electrodes in electrolyte solutions: I. capacitance effects. Electrochim Acta 8:751–780
Chen L, Lasia A (1993) Ni-Al powder electrocatalyst for hydrogen evolution. J Electrochem Soc 140:2464–2473
Hitz C, Lasia A (2001) Experimental study and modeling of impedance of the her on porous Ni electrodes. J Electroanal Chem 500:213–222
Jurczakowski R, Hitz C, Lasia A (2004) Impedance of porous Au based electrodes. J Electroanal Chem 572:355–366
Valek L, Metikos-Hukovic M, Grubac Z (2006) Impedance spectroscopy characterization of the electrodeposited Ni-15Mo catalyst designed for the HER in acid solution: modified porous model. J New Mat Electr Sys 9:145–153
Burg GJ, Eeden ALG, Sluyters-Rehbach M, Sluyters JH (1984) The analysis of electrode impedances complicated by the presence of a constant phase element. J Electroanal Chem 176:275–295
Acknowledgments
M.E. Melo Jorge thanks Fundação para a Ciência e Tecnologia (FCT) for funding (UID/MULTI/00612/2013). Acknowledgments are also due to Laboratório Nacional de Energia e Geologia (LNEG) in the framework of the MESOPOROUS project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Sá, A.I., Rangel, C.M. & Jorge, M.E.M. Electrochemical behavior of europium perovskites (Ca0.6Eu0.4MnO3) in alkaline aqueous media. J Solid State Electrochem 20, 1713–1722 (2016). https://doi.org/10.1007/s10008-016-3184-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-016-3184-9