Abstract
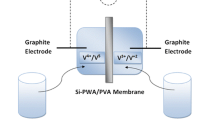
An ion exchange membrane (IEM) usually serves as a separator between the two half-cells and provides an ionic conduction path in redox flow batteries. The new vanadium solid-salt battery (VSSB) presents higher energy density than the traditional vanadium redox flow batteries (VRFBs). However, present IEMs are based on very expensive Nafion® membranes. In pursuit of lower cost, a membrane from sulfonated polystyrene (PE-01) is used for VSSB. In comparison with the traditional Nafion® 1135, PE-01 shows high energy efficiency with good cycling performance at current densities less than 10 mA cm−2. This suggests that sulfonated polystyrene membrane is a promising candidate as separator for VSSB.






Similar content being viewed by others
References
Sum E, Rychcik M, Skyllas-Kazacos M (1985) Investigation of the V(V)/V(IV) system for use in the positive half-cell of a redox battery. J Power Sources 16:85–95
Sum E, Skyllas-Kazacos M (1985) A study of the V(II)/V(III) redox couple for redox flow cell applications. J Power Sources 15:179–190
Skyllas-Kazacos M, Rychcik M, Robins RG, Fane AG, Green MA (1986) New all-vanadium redox flow cell. J Electrochem Soc 133:1057–1058
Li XF, Zhang HM, Mai ZS, Zhang HZ, Vankelecom I (2011) Ion exchange membranes for vanadium redox flow battery (VRB) applications. Energy Environ Sci 4:1147–1160
Wu XW, Liu J, Xiang XJ, Zhang J, Hu JP, Wu YP (2014) Electrolytes for vanadium redox flow batteries. Pure Appl Chem 86:1365–3075
Liu J, Hu J, Deng Q, Mo J, Xie H, Liu Z, Xiong Y, Wu X, Wu Y (2015) Aqueous rechargeable batteries for large-scale energy storage. Isr J Chem 55:521–536
Wu X, Liu J, Xie H, Xiong Y, Wu Y, Zhou Q (2014) Latest advance on carbon electrode materials for all vanadium redox flow battery. Sci Sin Chim 44:1280–1288
Skyllas-Kazacos M, Kazacos G, Poon G, Verseema H (2010) Recent advances with UNSW vanadium-based redox flow batteries. Int J Energy Res 34:182–189
Agar E, Benjamin A, Dennison CR, Chen D, Hickner MA, Kumbur EC (2014) Reducing capacity fade in vanadium redox flow batteries by altering charging and discharging currents. J Power Sources 246:767–774
Winardi S, Raghu SC, Oo MO, Yan QY, Wai N, Lim TM, Skyllas-Kazacos M (2014) Sulfonated poly (ether ether ketone)-based proton exchange membranes for vanadium redox battery applications. J Membr Sci 450:313–322
Park SK, Shim J, Yang JH, Jin CS, Lee BS, Lee YS, Shin KH, Jeon JD (2014) The influence of compressed carbon felt electrodes on the performance of a vanadium redox flow battery. Electrochim Acta 116:447–452
Yamamura T, Wu X, Ohta S, Shirasaki K, Sakuraba H, Satoh I, Shikama T (2011) Vanadium solid-salt battery: solid state with two redox couples. J Power Sources 196:4003–4011
Sacco A, Bella F, De La Pierre S, Castellino M, Bianco S, Bongiovanni R, Pirri CF (2015) Electrodes/electrolyte interfaces in the presence of a surface-modified photopolymer electrolyte: application in dye-sensitized solar cells. ChemPhysChem 16:960–969
Li MX, Wang XW, Yang YQ, Chang Z, Wu YP, Holze R (2015) A dense cellulose-based membrane as a renewable host for gel polymer electrolyte of lithium ion batteries. J Membr Sci 476:112–118
Tsao CH, Kuo PL (2015) Poly(dimethylsiloxane) hybrid gel polymer electrolytes of a porous structure for lithium ion battery. 289:36–42
Tabe Y, Aoyama Y, Kadowaki K, Suzuki K, Chikahisa T (2015) Impact of micro-porous layer on liquid water distribution at the catalyst layer interface and cell performance in a polymer electrolyte membrane fuel cell. J Power Sources 287:420–430
Schwenzer B, Zhang J, Kim S, Li L, Liu J, Yang Z (2011) Membrane development for vanadium redox flow batteries. ChemSusChem 4:1388–1406
Teng XG, Dai JiC SJ (2015) Effects of different kinds of surfactants on Nafion membranes for all vanadium redox flow battery. J Solid State Electrochem 19:1091–1101
Li JC, Zhang YP, Wang L (2015) Preparation and characterization of sulfonated polyimide/TiO2 composite membrane for vanadium redox flow battery. J Solid State Electrochem 18:729–737
Wu X, Hu J, Liu J, Zhou Q, Zhou W, Li H, Wu Y (2014) Ion exchange membranes for vanadium redox flow batteries. Pure Appl Chem 86:633–649
Sukkar T, Skyllas-Kazacos M (2004) Membrane stability studies for vanadium redox cell applications. J Appl Electrochem 34:137–145
Chen D, Hickner M, Agar E, Kumbur EC (2013) Optimizing membrane thickness for vanadium redox flow batteries. J Membr Sci 437:108–113
Kim J-G, Lee S-H, Choi S-I, Jin C-S, Kim J-C, Ryu C-H, Hwang G-J (2010) Application of Psf–PPSS–TPA composite membrane in the all-vanadium redox flow battery. J Ind Eng Chem 16:756–762
Wei XL, Nie ZM, Luo QT, Li B, Sprenkle V, Wang W (2013) Polyvinyl chloride/silica nanoporous composite separator for all-vanadium redox flow battery applications. J Electrochem Soc 160:A1215–A1218
Leung PK, Xu Q, Zhao TS, Zeng L, Zhang C (2013) Preparation of silica nanocomposite anion-exchange membranes with low vanadium-ion crossover for vanadium redox flow batteries. Electrochim Acta 105:584–592
Zhang HZ, Zhang HM, Li XF, Mai ZS, Wei WP (2012) Silica modified nanofiltration membranes with improved selectivity for redox flow battery application. Energy Environ Sci 5:6299–6303
Kim YW, Lee DK, Lee KJ, Kim JH (2008) Single-step synthesis of proton conducting poly(vinylidene fluoride) (PVDF) graft copolymer electrolytes. Eur Polym J 44:932–939
Zhang SH, Zhang BG, Zhao GF, Jian XG (2014) Anion exchange membranes from brominated poly(aryl ether ketone) containing 3,5-dimethyl phthalazinone moieties for vanadium redox flow batteries. J Mater Chem A 2:3083–3091
Zheng Q, Zhang HM, Xing F, Ma XK, Li XF, Ning GL (2014) A three-dimensional model for thermal analysis in a vanadium flow battery. Appl Energy 113:1675–1685
Ran J, Wu L, Wei B, Chen Y, Xu T (2014) Simultaneous enhancements of conductivity and stability for anion exchange membranes (AEMs) through precise structure design. Sci Rep 4:6486(1-5)
Kreuer KD (1996) Proton conductivity: materials and applications. Chem Mater 8:610–641
Sang SB, Huang HL, Wu QM (2008) An investigation on ion transfer resistance of cation exchange membrane/solution interface. Colloids Surf A Physicochem Eng Asp 315:98–102
Tian B, Yan CW, Wang FH (2004) Proton conducting composite membrane from daramic/nafion for vanadium redox flow battery. J Membr Sci 234:51–54
Acknowledgments
This work was supported by the National Distinguished Youth Scientists Project of China (No. 51425301), Hunan Provincial Natural Science Foundation of China (2015JJ3074), Science and Technology Project of Changsha (KL403147-11), and postdoctoral fund of Hunan Agricultural University (129263).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Dedicated to José H. Zagal on the occasion of his 65th birthday in appreciation of his contributions to electrocatalysis and to the general development of our electrochemical science in Chile and beyond.
Rights and permissions
About this article
Cite this article
Wang, Z., Hu, J., Wu, X. et al. A membrane based on sulfonated polystyrene for a vanadium solid-salt battery. J Solid State Electrochem 20, 943–948 (2016). https://doi.org/10.1007/s10008-015-2931-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-015-2931-7