Abstract
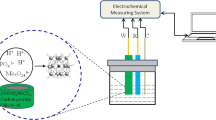
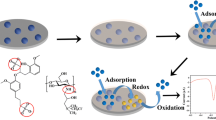
A new bifunctional disulfide- and tetrasulfide-bridged periodic mesoporous organosilica (PMO) with mercaptothiazoline ligand was synthesized and employed to prepare chemically modified carbon paste electrodes for Pb(II) detection in aqueous media by square wave adsorptive stripping voltammetry. To demonstrate the real interest of bifunctionalization, carbon paste electrodes were prepared with disulfide- and tetrasulfide-bridged PMO without mercaptothiazoline ligand. Results showed the importance of bifunctionalization of PMOs to improve the sensitivity for the determination of Pb(II) in water samples. To achieve the most accurate and sensitive Pb(II) measurements, optimization of the operating parameters in preconcentration and detection steps was performed. Finally, the modified carbon paste electrode prepared with bifunctional disulfide-bridged PMO with mercaptothiazoline ligand was applied to determine Pb(II) in different water samples without any pretreatment. Using this electrode, the optimal operating conditions were 120 s of electrolysis time in HCl 0.4 M. In these conditions, the voltammetric signal increased linearly with the preconcentration time from 1 to 10 min. Under optimized conditions, the linear range was 2–100 μg/L (R 2 = 0.9943) with a detection limit of 0.5 μg/L (for 5-min preconcentration time). Good reproducibility was achieved on both single and equally prepared electrodes. The accuracy of the method was validated by analysing Pb(II) in different drinking and natural water samples, with spiked recoveries in the range of 95–105 ± 10 %. The results demonstrated that the prepared electrochemical sensor exhibited selectivity, fast response time and exceptional long-time stability.









Similar content being viewed by others
References
World standards for allowable levels of lead in water (2014) Lead free water http://www.leadfreewater.com/world-standards/. Accessed 20 Oct 2014
Wang J (2000) Analytical electrochemistry. Willey, New York
Wang J (1985) Stripping analysis. VCH, Florida
Bellido-Milla D, Cubillana-Aguilera L, El Kaoutit M, Hernández-Artiga MP, de Cisneros Hidalgo-Hidalgo JL, Naranjo-Rodriguez I, Palacios Santander JM (2013) Anal Bional Chem 405:3525–3539
Roa Morales G, Ramírez Silva T, Galicia L (2003) J Solid State Electrochem 7:355–360
Cao LY, Jia JB, Wang ZH (2008) Electrochim Acta 53:2177–2182
Guo JX, Chai YQ, Yuan R, Song ZJ, Zou ZF (2011) Sensors Actuators B 155:639–645
Salmanipour A, Ali Taher M (2011) J Solid State Electrochem 15:2695–2702
Nguyen PKQ, Lunsford SK (2012) Talanta 101:110–121
Dai P, Yang Z (2012) Microchim Acta 176:109–115
Wang Y, Wu Y, Xie J, Hu X (2013) Sensors Actuators B 177:1161–1166
Morante-Zarcero S, Sánchez A, Fajardo M, del Hierro I, Sierra I (2010) Microchim Acta 169:57–64
Sánchez A, Morante-Zarcero S, Pérez-Quintanila D, del Hierro I, Sierra I (2013) J Electroanal Chem 689:76–82
Walcarius A, Etienne M, Sayen S, Lebeau B (2003) Electroanalysis 15:414–421
Sierra I, Pérez-Quintanilla D (2013) Chem Soc Rev 42:3792–3807
Walcarius A (2005) C R Chimie 8:693–712
Walcarius A (2008) Electroanalysis 20:711–738
Inagaki S, Guan S, Fukushima Y, Ohsuna T, Terasaki O (1999) J Am Chem Soc 121:9611–9614
Mizoshita N, Tani T, Inagaki S (2011) Chem Soc Rev 40:789–800
Asefa T, Kruk M, MacLachlan MJ, Coombs N, Grondey H, Jaroniec M, Ozin GA (2001) J Am Chem Soc 123:8520–8530
Zhang W-H, Daly B, O’Callaghan J, Zhang L, Shi J-L, Li C, Morris MA, Holmes JD (2005) Chem Mater 17:6407–6415
Zhan L, Zhang W, Shi J, Hua Z, Li Y, Yan J (2003) Chem Comm 210–215
Liu J, Yang J, Yang Q, Wang G, Li Y (2005) Adv Funct Mater 15:1297–1302
Hao N, Han L, Yang Y, Wang H, Webley PA, Zhao D (2010) Appl Surf Sci 256:5334–5342
Pérez-Quintanilla D, del Hierro I, Carrillo-Hermosilla F, Fajardo M, Sierra I (2006) Anal Bional Chem 384:827–837
Yantasee W, Lin Y, Zemanian TS, Fryxell GE (2003) Analyst 128:467–472
Yantasee W, Lin Y, Fryxell GE, Busche BJ (2004) Anal Chim Acta 502:207–212
Yantasee W, Fryxell GE, Conner MM, Lin Y (2005) J Nanosci Nanotech 5:15371540
Cesarino I, Marino G, Matos JR, Cavalheiro ETG (2008) Talanta 75:15–21
Acknowledgments
Authors thank financial support from CAM - European FEDER Program (Project S2013/ABI-3028)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morante-Zarcero, S., Pérez-Quintanilla, D. & Sierra, I. A disposable electrochemical sensor based on bifunctional periodic mesoporous organosilica for the determination of lead in drinking waters. J Solid State Electrochem 19, 2117–2127 (2015). https://doi.org/10.1007/s10008-015-2889-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-015-2889-5