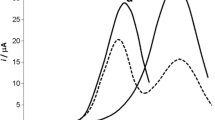
Abstract
The purpose of this paper is to propose mathematical modeling of different kinds of electrochemical processes. Electrooxidation of nitrite ions on some types of electrodes (glassy carbon, screen-printed carbon-containing, gold, and gold nanoparticles) and oxidation of ascorbic acid on gold electrodes nonmodified and modified by gold nanoparticles were used for the comparison of calculated and experimental data. It is shown that in each case, a specific shape of the voltammogram helps to identify the mechanism of the process. The comparison of the theoretical and experimental data shows that the process of oxidation of nitrite ions causes passivation of the electrode by adsorbed products of electrooxidation, and nanoeffects are not observed at that. Nanoeffects were observed in the oxidation of ascorbic acid on gold nanoparticles localized on gold electrode.









Similar content being viewed by others
References
Tau P, Nyokong T (2007) Electrocatalytic oxidation of nitrite by tetra-substituted oxotitanium(IV) phthalocyanines adsorbed or polymerised on glassy carbon electrode. J Electroanal Chem 611(1–2):10–18
Spataru N, Rao TN, Tryk DA, Fujishima A (2001) Determination of nitrite and nitrogen oxides by anodic voltammetry at conductive diamond electrodes. J Electrochem Soc 148(3):E112–E117
Kalimuthu P, John SA (2009) Highly sensitive and selective amperometric determination of nitrite using electropolymerized film of functionalized thiadiazole modified glassy carbon electrode. Electrochem Commun 11(5):1065–1068
Wu ZF, Ma YS, Zhang YL, Xu LS, Chen BH, Yuan Q, Huang WX (2012) Adsorption and surface reaction of NO2 on a stepped Au (997): surface enhanced reactivity of low-coordinated Au atoms. J Phys Chem C 116(5):3608–3617
Wickham DT, Banse BA, Koel BE (1990) Adsorption of nitrogen dioxide on polycrystalline gold. Catal Lett 6:163–172
Wang Y, Ward KR, Laborda E, Salter C, Crossley A, Jacobs RMJ, Compton RG (2013) A joint experimental and computational search for authentic nano-electrocatalytic effects: electrooxidation of nitrite and L-ascorbate on gold nanoparticle-modified glassy carbon electrodes. Small 9(3):478–486
Wang Y, Laborda E, Compton RG (2012) Electrochemical oxidation of nitrite: kinetic, mechanistic and analytical study by square wave voltammetry. J Electroanal Chem 670:56–61
Li J (2009) Electrocatalytic oxidation of nitrite at gold nanoparticle-polypyrrole nanowire modified glassy carbon electrode. Chin J Chem 27(12):2373–2378
Huang X, Li YX, Chen YL, Wang L (2008) Electrochemical determination of nitrite and iodate by use of gold nanoparticles/poly(3-methylthiophene) composites coated glassy carbon electrode. Sens Actuators, B 134(2):780–786
Wang X, Li H, Wu M, Ge SL, Zhu Y, Wang QJ, He PG, Fang YZ (2013) Simultaneous electrochemical determination of sulphite and nitrite by a gold nanoparticle/graphene-chitosan modified electrode. Chin J Anal Chem 41(8):1232–1237
Cui YP, Yang CZ, Zeng W, Oyama M, Pu WH, Zhang JD (2007) Electrochemical determination of nitrite using a gold nanoparticles-modified glassy carbon electrode prepared by the seed-mediated growth technique. Anal Sci 23(12):1421–1425
Zhang JD, Oyama M (2005) Gold nanoparticle arrays directly grown on nanostructured indium tin oxide electrodes: characterization and electroanalytical application. Anal Chim Acta 540(2):299–306
Brainina KZ, Galperin LG, Galperin AL (2010) Mathematical modeling and numerical simulation of metal nanoparticles electrooxidation. J Solid State Electrochem 14(6):981–988
Carslow HS, Jaeger JC (1959) Conduction of heat in solids, 2nd edn. Oxford University Press, USA
BabenkoYuI (2009) Metod drobnogo differencirovaniya v prikladnyh zadachah teorii teplomassoobmena. Professional, Sankt-Peterburg
Turkevich J, Stevenson PC, Hillier J (1953) The formation of colloidal gold. J Phys Chem 57:670–673
Jiang YN, Luo HQ, Li NB (2007) Determination of nitrite with a nano-gold modified glassy carbon electrode by cyclic voltammetry. Int J Environ Anal Chem 87(4):295–306
Piela B, Wrona PK (2002) Oxidation of nitrites on solid electrodes—I. Determination of the reaction mechanism on the pure electrode surface. J Electrochem Soc 149(2):E55–E63
Rohani T, Taher MA (2009) A new method for electrocatalytic oxidation of ascorbic acid at the Cu(II) zeolite-modified electrode. Talanta 78(3):743–747
Sivanesan A, Kannan P, John SA (2007) Electrocatalytic oxidation of ascorbic acid using a single layer of gold nanoparticles immobilized on 1,6-hexanedithiol modified gold electrode. Electrochim Acta 52(28):8118–8124
Kreshkov AP (ed) (1971) Osnovy analiticheskoy khimii, vol 2. Khimiâ, Moskva, p 287
Nikolskiî BP (ed) (1966) Spravochnik khimika, vol 1. Khimiâ, Moskva, p 1006
Brainina KZ, Galperin LG, Vikulova EV, Galperin AL (2013) The effect of the system polydispersity on voltammograms of nanoparticles electrooxidation. J Solid State Electrochem 17(1):43–53
Brainina KZ, Galperin LG, Vikulova EV, Stozhko NY, Murzakaev AM, Timoshenkova OR, Kotov YA (2011) Gold nanoparticles electrooxidation: comparison of theory and experiment. J Solid State Electrochem 15(5):1049–1056
Karimi MA, Hasheminasab M (2013) Determination of iron(III) in N-methyldiethanolamine media utilized in sweetening plant of gas treating industry by using self-assembled monolayer on gold electrode. Int J Electrochem Sci 8(4):4560–4570
Nikolskiî NP (ed) (1965) Spravochnik khimika, vol 1. Khimiâ, Moskva, p 383
Kalimuthu P, John SA (2008) Size dependent electrocatalytic activity of gold nanoparticles immobilized onto three dimensional sol-gel network. J Electroanal Chem 617(2):164–170
Acknowledgments
The authors express their deep gratitude to the financial support of the RFBR (Project # 13-03-00285_a and # 13-00-14148_Ir) and the Ministry of Education and Science of RF (Project # 1458 in the framework of the assignment 4.1458.2014/k).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brainina, K.Z., Galperin, L.G., Bukharinova, M.A. et al. Mathematical modeling and experimental study of electrode processes. J Solid State Electrochem 19, 599–606 (2015). https://doi.org/10.1007/s10008-014-2642-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-014-2642-5