Abstract
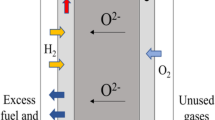
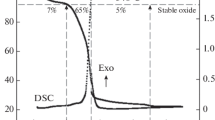
Highly pure Ba0.5Sr0.5Co0.8Fe0.2O3 − δ (BSCF) perovskite nanocompound was synthesized via glycine-nitrate process (GNP) and coprecipitation-azeotropic distillation method (ADM), respectively. Compared to ADM, GNP was proved to facilitate the synthesis of BSCF powder with small crystal and particle sizes as well as large surface area. BSCF material was employed as the cathode of solid oxide fuel cell (SOFC) incorporating with Sm0.2Ce0.8O1.9 (SDC) electrolyte. Linear sweep voltammetry (LSV) and electrochemical impedance spectroscopy (EIS) tests indicated that the GNP powder-based cathode not only exhibited high electrocatalytic activity but also possessed low oxygen diffusion resistance due to the high porosity. As a result, the cell with GNP powder-based cathode showed a power density of 0.35 W cm−2 when the voltage was 0.7 V at 650 °C. Furthermore, the cathode performance was significantly improved at the high current density condition.







Similar content being viewed by others
References
Zhang L, Chen FL, Xia CR (2010) Spin-coating derived solid oxide fuel cells operated at temperatures of 500 °C and below. Int J Hydrog Energy 35:13262–13270
Shi Z, Sun WP, Liu W (2014) Synthesis and characterization of BaZr0.3Ce0.5Y0.2 − x Yb x O3 − δ proton conductor for solid oxide fuel cells. J Power Sources 245:953–957
Chen YH, Wei YJ, Zhong HH, Gao JF, Liu XQ, Meng GY (2007) Synthesis and electrical properties of Ln0.6Ca0.4FeO3 − δ (Ln = Pr, Nd, Sm) as cathode materials for IT-SOFC. Ceram Int 33:841–849
Mori T, Buchanan R, Ou DR, Ye F, Kobayashi T, Kim JD, Zou J, Drennan J (2008) Design of nanostructured ceria-based solid electrolytes for development of IT-SOFC. J Solid State Electrochem 12:2897–2907
Chen JY, Rebello J, Vashook V, Trots DM, Wang SR, Wen TL, Zosel J, Guth U (2011) Thermal stability, oxygen non-stoichiometry and transport properties of LaNi0.6Fe0.4O3. Solid State Ionics 192:424–430
Kim JH, Irvine JTS (2012) Characterization of layered perovskite oxides NdBa1 − x Sr x Co2O5 + δ (x = 0 and 0.5) as cathode materials for IT-SOFC. Int J Hydrog Energy 37:5920–5929
Wang ZH, Xu C, Lou ZL, Qiao JS, Ren BY, Sun KN (2013) Preparation and characterization of silver-modified La0.8Sr0.2MnO3 cathode powders for solid oxide fuel cells by chemical reduction method. Int J Hydrog Energy 38:1074–1081
Lu J, Yin YM, Ma ZF (2013) Preparation and characterization of new cobalt-free cathode Pr0.5Sr0.5Fe0.8Cu0.2O3 − δ for IT-SOFC. Int J Hydrog Energy 38:10527–10533
Pelosato R, Cristiani C, Dotelli G (2013) Co-precipitation synthesis of SOFC electrode materials. Int J Hydrog Energy 38:480–491
Shao ZP, Haile SM (2004) A high-performance cathode for the next generation of solid-oxide fuel cells. Nature 431:170–173
Duan ZS, Yang M, Yan AY, Hou ZF, Dong YL, Chong Y, Cheng MJ, Yang WS (2006) Ba0.5Sr0.5Co0.8Fe0.2O3 − δ as a cathode for IT-SOFCs with a GDC interlayer. J Power Sources 160:57–64
Liu BW, Zhang Y, Zhang LM (2009) Oxygen reduction mechanism at Ba0.5Sr0.5Co0.8Fe0.2O3 − δ cathode for solid oxide fuel cell. Int J Hydrog Energy 34:1008–1014
Hardy JS, Templeton JW, Edwards DJ, Lu ZG, Stevenson JW (2012) Lattice expansion of LSCF-6428 cathodes measured by in situ XRD during SOFC operation. J Power Sources 198:76–82
Švarcová S, Wiik K, Tolchard J, Bouwmeester HJM, Grande T (2008) Structural instability of cubic perovskite Ba x Sr1 − x Co1 − y FeyO3 − δ . Solid State Ionics 178:1787–1791
Yan AY, Liu B, Dong YL, Tian ZJ, Wang DZ, Cheng MJ (2008) A temperature programmed desorption investigation on the interaction of Ba0.5Sr0.5Co0.8Fe0.2O3 − δ perovskite oxides with CO2 in the absence and presence of H2O and O2. Appl Catal B Environ 80:24–31
Schmale K, Barthel J, Bernemann M, Grünebaum M, Koops S, Schmidt M, Mayer J, Wiemhöfer HD (2013) AFM investigations on the influence of CO2 exposure on Ba0.5Sr0.5Co0.8Fe0.2O3 − δ . J Solid State Electrochem 17:2897–2907
Bucher E, Egger A, Caraman GB, Sitte W (2008) Stability of the SOFC cathode material (Ba, Sr)(Co, Fe)O3 in CO2-containing atmospheres. J Electrochem Soc 155:B1218–B1224
Huang W, Shuk P, Greenblatt M (1997) Properties of sol-gel prepared Ce1 − x Sm x O2 − x/2 solid electrolytes. Solid State Ionics 100:23–27
Kong JR, Zhang Y, Deng CS, Xu JM (2009) Synthesis and electrochemical properties of LSM and LSF perovskites as anode materials for high temperature steam electrolysis. J Power Sources 186:485–489
Zhao Z, Liu L, Zhang XM, Tu BF, Ou DR, Cheng MJ (2012) Carbonates formed during BSCF preparation and their effects on performance of SOFCs with BSCF cathode. Int J Hydrog Energy 37:19036–19044
Acknowledgments
This work was financially supported by Natural Science Foundation of China (21276028, 51102024), Natural Science Foundation of Hunan Province (12JJ6015, 12JJ3039), as well as Construct Program of Key Discipline in Hunan Province.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, P., Kong, J., Liu, Q. et al. Relationship between powder structure and electrochemical performance of Ba0.5Sr0.5Co0.8Fe0.2O3 − δ cathode material. J Solid State Electrochem 18, 1513–1517 (2014). https://doi.org/10.1007/s10008-013-2374-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-013-2374-y