Abstract
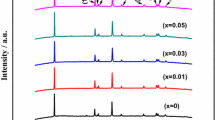
LiNi1/3Co1/3Mn1/3O2 cathode materials for the application of lithium ion batteries were synthesized by carbonate co-precipitation routine using different ammonium salt as a complexant. The structures and morphologies of the precursor [Ni1/3Co1/3Mn1/3]CO3 and LiNi1/3Co1/3Mn1/3O2 were investigated through X-ray diffraction, scanning electron microscope, and transmission electron microscopy. The electrochemical properties of LiNi1/3Co1/3Mn1/3O2 were examined using charge/discharge cycling and cyclic voltammogram tests. The results revealed that the microscopic structures, particle size distribution, and the morphology properties of the precursor and electrochemical performance of LiNi1/3Co1/3Mn1/3O2 were primarily dependent on the complexant. Among all as-prepared LiNi1/3Co1/3Mn1/3O2 cathode materials, the sample prepared from Na2CO3–NH4HCO3 routine using NH4HCO3 as the complexant showed the smallest irreversible capacity of 19.5 mAh g−1 and highest discharge capacity of 178.4 mAh g−1 at the first cycle as well as stable cycling performance (98.7% of the initial capacity was retained after 50 cycles) at 0.1 C (20 mA g−1) in the voltage range of 2.5–4.4 V vs. Li+/Li. Moreover, it delivered high discharge capacity of over 135 mAh g−1 at 5 C (1,000 mA g−1).













Similar content being viewed by others
References
Ohzuku T, Makimura Y (2001) Chem Lett 30:642–643
MacNeil DD, Lu Z, Dahn JR (2002) J Electrochem Soc 149:A1332–A1336
Cheralathan KK, Kang NY, Park HS, Lee YJ, Choi WC, Ko YS, Park YK (2010) J Power Sources 195:1486–1494
Park SH, Shin HS, Myung ST, Yoon CS, Amine K, Sun YK (2005) Chem Mater 17:6–8
Sun YK, Myung ST, Park BC, Prakash J, Belharouak I, Amine K (2009) Nat Mater 8:320–324
Shaju KM, Bruce PG (2006) Adv Mater 18:2330–2334
Li DC, Muta T, Zhang LQ, Yoshio M, Noguchi H (2004) J Power Sources 132:150–155
Kim JM, Chung HT (2004) Electrochim Acta 49:937–944
Shizuka K, Kobayashi T, Okahara K, Okamoto K, Kanzaki S, Kanno R (2005) J Power Sources 146:589–593
Luo XF, Wang XY, Liao L, Gamboa S, Sebastian PJ (2006) J Power Sources 158:654–658
Shaju KM, Subba Rao GV, Chowdari BVR (2002) Electrochim Acta 48:145–151
Zhang XY, Jiang WJ, Mauger A, Lu Q, Gendrond F, Julien CM (2010) J Power Sources 195:1292–1301
Zhang S (2007) Electrochim Acta 52:7337–7342
Deng C, Liu L, Zhou W, Sun K, Sun D (2008) Electrochim Acta 53:2441–2447
Lee M-H, Kanga Y-J, Myung S-T, Sun Y-K (2004) Electrochim Acta 50:939–948
Lavela P, Sanchez L, Tirado JL, Bach S, Pereira-Ramos JP (1999) J Power Sources 84:75–79
Yoncheva M, Stoyanova R, Zhecheva E, Alcántara R, Tirado JL (2009) J Alloys Compd 475:96–101
Park SH, Kang SH, Belharouak I, Sun YK, Amine K (2008) J Power Sources 177:177–183
Ren HB, Huang YH, Wang YH, Li ZJ, Cai P, Peng ZH, Zhou YH (2009) Mater Chem Phys 117:41–45
Zhang S, Deng C, Fu BL, Yang SY, Ma L (2010) Powder Technol 198:373–380
Zhang S, Deng C, Yang SY, Niu H (2009) J Alloys Compd 484:519–523
Cho TH, Park SM, Yoshio M, Hirai T, Hideshima Y (2005) J Power Sources 142:306–312
He P, Wang HR, Qi L, Osaka T (2006) J Power Sources 160:627–632
Alexander LE (1969) X-ray diffraction methods in polymer science. Wiley, New York
Cho TH, Park SM, Yoshio M (2004) Chem Lett 33:704–705
Park SM, Cho TH, Yoshio M (2004) Chem Lett 33:748–749
Park KS, Cho MH, Jin SJ, Nahm KS (2004) Electrochem Solid-State Lett 7:A239–A241
Rougier A, Gravereau P, Delmas C (1996) J Electrochem Soc 143:1168–1175
Shin HS, Park SH, Bae YC, Sun YK (2005) Solid State Ionics 176:2577–2581
Reimers JN, Rossen E, Jones CD, Dahn JR (1993) Solid State Ionics 61:335–344
Ohzuku T, Ueda A, Nagayama M (1993) J Electrochem Soc 140:1862–1869
Wang ZX, Sun YC, Chen LQ, Huang XJ (2004) J Electrochem Soc 151:A914–A921
Paulsen JM, Thomas CL, Dahn JR (2000) J Electrochem Soc 147:861–868
Ohzuku T, Ueda A, Nagayama M, Iwakoshi Y, Komori H (1993) Electrochim Acta 38:1159–1167
Madhavi S, Subba Rao GV, Chowdari BVR, Li SFY (2001) J Electrochem Soc 148:A1279–A1286
Armstrong AR, Robertson AD, Gitzendanner R, Bruce PG (1999) J Solid State Chem 145:549–556
Kajiyama A, Takada K, Inada T, Kouguchi M, Kondo S, Watanabe M (2001) J Electrochem Soc 148:A981–A983
Acknowledgments
This work is funded by the National Natural Science Foundation of China under project no. 20871101, Scientific Research Fund of Hunan Provincial Education Department no. 09C947, Key Project of Science and Technology Department of Hunan Province Government under project no. 2009WK2007, and Colleges and Universities in Hunan Province plans to graduate research and innovation under project no. CX2009B133.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, S., Wang, X., Chen, Q. et al. Effects of complexants on [Ni1/3Co1/3Mn1/3]CO3 morphology and electrochemical performance of LiNi1/3Co1/3Mn1/3O2 . J Solid State Electrochem 16, 481–490 (2012). https://doi.org/10.1007/s10008-011-1356-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-011-1356-1