Abstract
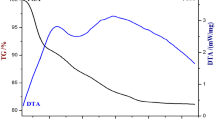
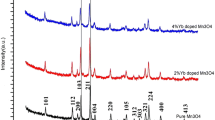
Manganese–vanadium oxide had been synthesized by a novel simple precipitation technique. Scanning electron microscopy, X-ray diffraction, Brunauer–Emmett–Teller, thermogravimetric analysis/differential scanning calorimetry, and X-ray photoelectron spectroscopy were used to characterize Mn–V binary oxide and δ-MnO2. Electrochemical capacitive behavior of the synthesized Mn–V binary oxide and δ-MnO2 was investigated by cyclic voltammetry, galvanostic charge–discharge curve, and electrochemical impedance spectroscope methods. The results showed that, by introducing V into δ-MnO2, the specific surface area of the mixed oxide increased due to a formation of small grain size. The specific capacitance increased from 166 F g−1 estimated for MnO2 to 251 F g−1 for Mn–V binary oxide, and the applied potential window extended to −0.2–1.0 V (vs. saturated calomel electrode). Through analysis, it is suggested that the capacitance performance of Mn–V binary oxide materials may be improved by changing the following three factors: (1) small grain and particle size and large activity surface area, (2) appropriate amount of lattice water, and (3) chemical state on the surface of MnO2 material.









Similar content being viewed by others
References
Bagotsky VS (2006) Fundamentals of electrochemistry. Wiley, New York
Lazzari M, Soavi F, Mastragostino M (2009) J Electrochem Soc 156:A661–A666
Changa KH, Hua CC, Chou CY (2009) Electrochim Acta 54:978–983
Athouél L, Moser F, Gugas R, Crosnier O, Blanger D, Brousse T (2008) J Phys Chem C 112:7270–7277
Chen L, Yuan C, Gao B, Chen S, Zhang X (2009) J Solid State Electrochem 13:1925–1933
Qu QT, Shi Y, Li LL, Guo WL, Wu YP, Zhang HP, Guan SY, Holze R (2009) Electrochem Comm 11:1325–1328
Xiao W, Xia H, Fuh JYH, Lu L (2009) J Power Sources 193:935–938
Reddy RN, Reddy RG (2003) J Power Sources 124:330–337
Devaraj S, Munichandraiah N (2008) J Solid State Electrochem 12:207–211
Yuan AB, Wang ML, Wang YQ, Hu J (2009) Electrochim Acta 54:1021–1024
Ghaemi M, Ataherian F, Zolfaghari A, Jafari SM (2008) Electrochim Acta 53:4607–4614
Kim H, Popov BN (2003) J Electrochem Soc 150:D56–D62
Lee MT, Chang JK, Hsieh YT, Tsai WT (2008) J Power Sources 185:1550–1556
Machefaux E, Brousse T, Bélanger D, Guyomard D (2007) J Power Sources 165:651–655
Nakayama M, Tanaka A, Konishi S, Ogura K (2004) J Mater Res 19:1509–1515
Chen LM, Lai QY, Hao YJ, Zhao Y, Ji XY (2009) J Alloys Compd 467:465–471
Zolfaghari A, Ataherian F, Ghaemi M, Gholami A (2007) Electrochim Acta 52:2806–2814
Ragupathy P, Park DH, Campet G, Vasan HN, Hwang SJ, Choy JH, Munichandraiah N (2009) J Phys Chem C 113:6303–6309
Cullity BD, Stock SR (2001) Element of X-ray diffraction. Prentice-Hall, New Jersey
Khyzhuna OY, Strunskus T, Grünert W, Wöll C (2005) J Electron Spectrosc Relat Phenom 149:45–50
Yuan A, Wang X, Wang Y, Hu J (2009) Electrochim Acta 54:1021–1026
Donkova B, Mehandjiev D (2004) Thermochim Acta 421:141–149
Devaraj S, Munichandraian N (2007) J Electrochem Soc 154:A80–A88
Yuan A, Zhang QL (2006) Electrochem Commun 8:1173–1178
Xu Q, Zhao K, Gu C (2002) Chinese J Rare Metal 26:169–172
Toupin T, Brousse T, Bélanger D (2002) Chem Mater 14:3946–3952
Nagarajan N, Cheong M, Zhitomirsky I (2007) Mater Chem Phys 103:47–53
Chang JK, Chen YL, Tsai WT (2004) J Power Sources 135:344–353
Subramanian V, Zhu H, Vajtai R, Ajayan PM, Wei B (2005) J Phys Chem B 109:20207–20214
Chang JK, Tsai WT (2003) J Electrochem Soc 150:A1333–A1338
Wei W, Cui X, Chen W, Ivey DG (2009) J Power Sources 186:543–550
Yan D, Yan P, Cheng S, Chen J, Zhuo R, Feng J, Zhang G (2009) Cryst Growth Des 9:218–222
Strohmeier BR, Hercules DM (1984) J Phys Chem 88:4922–4929
Nguyen TD, Do TO (2009) Langmuir 25:5322–5332
Chigane M, Ishikawa M (2000) J Electrochem Soc 147:2246–2251
Chigane M, Ishikawa M, Izaki M (2001) J Electrochem Soc 148:D96–D101
Gamby J, Taberna PL, Simon P, Fauvarque JF, Chesneau M (2001) J Power Sources 101:109–116
Wu MS, Huang YA, Yang CH (2008) J Electrochem Soc 155:A798–A805
Devaraj S, Munichandraiah N (2008) J Phys Chem C 112:4406–4417
Chang JK, Huang CH, Lee MT, Tsai WT, Deng MJ, Sun IW (2009) Electrochim Acta 54:3278–3284
Ghodbane O, Pascal JL, Favier F (2009) Appl Mater Inter 1:1130–1139
Hu CC, Wu YT, Chang KH (2008) Chem Mater 20:2890–2894
Lin ML, Lo MY, Mou CY (2009) J Phys Chem C 113:16158–16168
Acknowledgment
Financial support for this work was provided by Major State Basic Research Development Program (no. 2008CB617502).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xie, X., Liu, W., Zhao, L. et al. Structural and electrochemical behavior of Mn–V oxide synthesized by a novel precipitation method. J Solid State Electrochem 14, 1585–1594 (2010). https://doi.org/10.1007/s10008-009-0987-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-009-0987-y