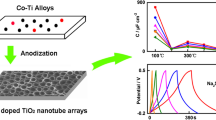
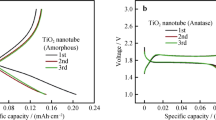
Abstract
TiO2 array film fabricated by potentiostatic anodization of titanium is characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), and charge–discharge measurements. The XRD results indicated that the TiO2 array is amorphous, and after calcination at 500 °C, it has the anatase form. The pore size and wall thickness of TiO2 nanotube arrays synthesized at different anodization voltages are highly dependent on the applied voltage. The electrochemical performance of the prepared TiO2 nanotube array as an electrode material for lithium batteries was evaluated by galvanostatic charge–discharge measurement. The sample prepared at 20 V shows good cyclability but low discharge capacity of 180 mA h cm−3, while the sample prepared at 80 V has the highest discharge capacity of 340 mA h cm−3.




Similar content being viewed by others
References
Croce F, Appetecchi GB, Persi L, Scrosati B (1998) Nature 394:456
Wang DH, Choi DW, Yang ZG, Viswanathan VV, Nie ZM, Song YJ, Zhang JG, Liu J (2008) Chem Mater 20:3435
Wagemaker M, Kearley GJ, van Well AA, Mutka H, Mulder FM (2003) J Am Chem Soc 125:840
Wang YQ, Hu GQ, Duan XF, Sun HL, Xue QK (2002) Chem Phys Lett 365:427
Yuan ZY, Colomer JF, Su BL (2002) Chem Phys Lett 363:362
Zhang XY, Yao BD, Zhao LX, Liang CH, Zhang LD, Mao YQ (2001) J Electrochem Soc 148:G398
Gong D, Grimes CA, Varghese OK, Gong D, Grimes CA, Varghese OK, Hu W, Singh RS, Chen Z, Dickey EC (2001) J Mater Res 16:3331
Sun L, Li J, Zhuang HF, Lai YK, Wang CL, Lin CJ (2007) Chinese J Inorg Chem 23:1841
Parkhutik VP, Shershulsky VI (1992) J Phys D: Appl Phys 25:1258
Gopal KM, Oomman KV, Maggie P, Karthik S, Craig AG (2006) Sol Energy Mater Sol Cells 90:2011
Maier J (2005) Nat Mater 4:805
Wang Y, Cao GZ (2006) Chem Mater 18:2787
Attia A, Zukalova M, Rathousky J, Zukal A, Kavan L (2005) J Solid State Electrochem 9:138
Moriguchi I, Hidaka R, Yamada H, Kudo T (2005) Solid State Ionics 176:2361
Acknowledgements
This work is financially supported by Science & Technology Commission of Shanghai Municipality (08DZ2270500) and National “973” Project (No. 2009CB220100).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, Z., Liu, Z., Jiang, R. et al. TiO2 nanotube array film prepared by anodization as anode material for lithium ion batteries. J Solid State Electrochem 14, 1045–1050 (2010). https://doi.org/10.1007/s10008-009-0910-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-009-0910-6