Abstract
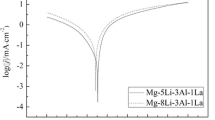
Mg–Li–Al–Sn and Mg–Li–Al–Sn–Ce alloys were prepared using a vacuum induction melting method. Their electrochemical oxidation behavior in NaCl solution was investigated by means of potentiodynamic and chronoamperometric measurements. The surface morphology after discharge was examined using scanning electron microscopy. Utilization efficiency was estimated with a mass-loss method. The results indicated that Mg–Li–Al–Sn has a higher discharge current density but lower utilization efficiency than Mg–Li–Al–Sn–Ce. The typical utilization efficiency after continuous discharging at constant potential of −1.0 for 2 h is 65% and 70% for Mg–Li–Al–Sn and Mg–Li–Al–Sn–Ce, respectively. The utilization efficiency decreased with the increase of anodic potential. Both alloys have similar self-discharge rate in NaCl solution at open-circuit potential.





Similar content being viewed by others
References
Medeiros MG, Bessette RR, Deschenes CM, Patrissi CJ, Carreiro LG, Tucker SP, Atwater DW (2004) J Power Sources 136:226–231. doi:10.1016/j.jpowsour.2004.03.024
Medeiros MG, Bessette RR, Deschenes CM, Atwater DW (2001) J Power Sources 96:236–239. doi:10.1016/S0378-7753(01)00500-6
Medeiros MG, Dow EG (1999) J Power Sources 80:78–82. doi:10.1016/S0378-7753(98)00253-5
Bessette RR, Medeiros MG, Patrissi CJ, Deschenes CM, LaFratta CN (2001) J Power Sources 96:240–244. doi:10.1016/S0378-7753(01)00492-X
Yang W, Yang S, Sun W, Sun G, Xin Q (2006) Electrochim Acta 52:9–14. doi:10.1016/j.electacta.2006.03.066
Cao D, Wu L, Wang G, Lv Y (2008) J Power Sources 183:799–804. doi:10.1016/j.jpowsour.2008.06.005
Hasvold O, Storkersen NJ, Forseth S, Lian T (2006) J Power Sources 162:935–942. doi:10.1016/j.jpowsour.2005.07.021
Hasvold O, Lian T, Haakaas E, Storkersen N, Perelman O, Cordier S (2004) J Power Sources 136:232–239. doi:10.1016/j.jpowsour.2004.03.023
Hamlen RP, Atwater DW in: D. Linden, and T.B. Reddy, (Eds.), Handbook of Batteries, McGraw-Hill. 381.
Udhayan R, Muniyandi N, Mathur PB (1992) Br Corros J 27:68–71
Sivashanmugam A, kumar TP, Renganathan NG, Gopukumar S (2004) J Appl Electrochem 34:1135–1139. doi:10.1007/s10800-004-2728-3
Cao D, Wu L, Sun Y, Wang G, Lv Y (2008) J Power Sources 177:624–630. doi:10.1016/j.jpowsour.2007.11.037
Acknowledgments
We gratefully acknowledge the financial support of this research by Heilongjiang Provincial Natural Science Foundation (ZJG2007-06-02), Heilongjiang Postdoc Foundation (LBH-Q06091), and Harbin Engineering University (HEUFT07030).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cao, D., Cao, X., Wang, G. et al. Electrochemical discharge performance of Mg-Li based alloys in NaCl solution. J Solid State Electrochem 14, 851–855 (2010). https://doi.org/10.1007/s10008-009-0865-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-009-0865-7