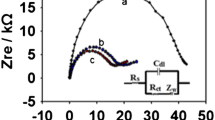
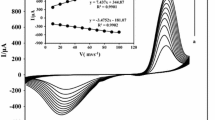
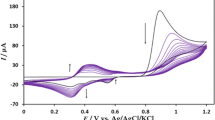
Abstract
A conductive carbon paste electrode (CPE) comprising a new zinc complex of [Zn(C11H15N3)2Cl2] and carbon powder was fabricated by the direct mixing method. The structure of [Zn(C11H15N3)2Cl2] was characterized by X-ray crystallography and elemental analysis. The electrochemical behavior and electrocatalysis of the new zinc complex modified CPE (Zn–CPE) have been studied in detail. This bulk-modified Zn–CPE displayed good electrocatalytic activity toward the reduction of the hydrogen peroxide and nitrite. The results are reproducible with a lower detection limit than reported currently using other modified electrodes. Also, this bulk-modified CPE exhibits remarkable long-term stability, simple preparation, and inexpensive material, which is important for practical application on electrochemistry sensors.




Similar content being viewed by others
References
Ngo HL, Hu AG, Lin WB (2004) J Mol Catal A 215:177
Sanchez C, Lebeau B, Chaput F, Boilet JP (2003) Adv Mater 15:1969. doi:10.1002/adma.200300389
Evans OR, Lin WB (2001) Chem Mater 13:3009. doi:10.1021/cm010427k
Jannasch P (2003) Curr Opin Colloid Interface Sci 8:96. doi:10.1016/S1359-0294(03)00006-2
Javaid A, Hughey MP, Varutbangkul V, Ford DM (2001) Membr Sci 187:141. doi:10.1016/S0376-7388(01)00341-6
Honma I, Nomura S, Nakajima HJ (2001) Membr Sci 185(1):83. doi:10.1016/S0376-7388(0000636-0
Sudik AC, Millward AR, Ockwig NW, Cote AP, Kim J, Yaghi OM (2005) J Am Chem Soc 127:7110. doi:10.1021/ja042802q
Rowsell JLC, Millward AR, Park KS, Yaghi OM (2004) J Am Chem Soc 126:5666. doi:10.1021/ja049408c
Cheung KC, Wong WL, Ma DL, Lai TS, Wong KY (2007) Coord Chem Rev 251:2367. doi:10.1016/j.ccr.2007.04.004
Abdollah S, Hussein M, Sajjad M (2006) Electrochem Commun 8:688. doi:10.1016/j.elecom.2006.02.019
Ruiperez J, Mendiola MA, Sevilla MT, Procopio JR, Hernandeza L (2002) Electroanalysis 14:532. doi:10.1002/1521-4109(200204)14:7/8<532::AID-ELAN532>3.0.CO;2-#
Wang XL, Wang EB, Hu CW (2001) Chem Lett 10:1030. doi:10.1246/cl.2001.1030
Wang XL, Zhang Q, Han ZB, Wang EB, Guo YQ, Hu CW (2004) J Electroanal Chem 563:221. doi:10.1016/j.jelechem.2003.09.015
Wang XL, Han ZB, Zhang H, Wang EB, Hu CW (2003) Electroanalysis 15:1460. doi:10.1002/elan.200302668
Chen SM (1998) J Electroanal Chem 457:23. doi:10.1016/S0022-0728(98)00143-0
Wang XL, Lin HY, Liu GC, Zhao HY, Chen BK (2008) J Organomet Chem 693:2767. doi:10.1016/j.jorganchem.2008.05.031
Wang XL, Zhao HY, Lin HY, Liu GC, Fang JN, Chen BK (2008) Electroanalysis 20:1055. doi:10.1002/elan.200704146
Li C, Cao R, O’Halloran KP, Ma H, Wu L (2008) Electrochim Acta 54:484. doi:10.1016/j.electacta.2008.07.039
Zhao Z, Zhou B, Su Z, Ma H, Li C (2008) Inorg Chem Commun 11:648. doi:10.1016/j.inoche.2008.02.032
Sheldrick GM (1990) Acta Crystallogr A 46:467. doi:10.1107/S0108767390000277
Sheldrick GM (1997) Program for crystal structure refinement, SHELXTL97. University of Gottingen, Germany
Wilson AJ (1992) International table for X-ray crystallography. Kluwer, Dordrecht
Lu J, Zhao K, Fang QR, Xu JQ, Yu JH, Zhang X, Bie HY, Wang TG (2005) Cryst Growth Des 5(3):1091. doi:10.1021/cg049637a
Gonzalez GLL, Kahlert H, Scholz F (2007) Electrochim Acta 52:1968. doi:10.1016/j.electacta.2006.08.006
Ounathan JNY, Wood KS, Meyer TJ (1992) Inorg Chem 31:3280. doi:10.1021/ic00041a022
Wang XL, Wang EB, Lan Y, Hu CH (2002) Electroanalysis 14:1116. doi:10.1002/1521-4109(200208)14:15/16<1116::AID-ELAN1116>3.0.CO;2-F
Huang X, Li YX, Chen YL, Wang L (2008) Sens Actuators B Chem 134:780. doi:10.1016/j.snb.2008.06.028
Acknowledgments
This work was supported by the Natural Science Foundation of Shandong Province (no. 2007BS04046), P. R. China.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
This page is unfortunately not in the Publisher's archive anymore:
Crystallographic data for the structural analysis have been deposited with the Cambridge Crystallographic Data Centre, CCDC reference number 710363. Copies of this information may be obtained free of charge from The Director, 12 Union Road, Cambridge, CB2 1EZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk or http://www.ccdc.cam.ac.uk). (CIF 6 kb)
Rights and permissions
About this article
Cite this article
Zhuang, R.R., Jian, F.F. Electrocatalysis for the hydrogen peroxide and nitrite at carbon paste electrode modified with a new zinc complex of 1-pentyl-1H-benzo[d][1,2,3]triazole. J Solid State Electrochem 14, 747–750 (2010). https://doi.org/10.1007/s10008-009-0844-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-009-0844-z