Abstract
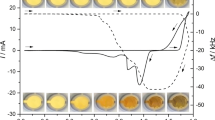
Electrochemical quartz crystal microbalance, combined with cyclic voltammetric, chronoamperometric, and potentiostatic measurements, was used to study electrodeposition/dissolution phenomena at a gold electrode in solutions containing Na2S. Spontaneous, open-circuit deposition processes as well as dissolution of the deposits in sulfide-free solutions have also been investigated. The potential range, scan rate, sulfide concentration, and pH have been varied. The results of the piezoelectric nanogravimetric studies are elucidated by a rather complex scheme involving underpotential deposition of sulfur at approximately −0.85 V vs. sodium calomel electrode, reductive dissolution of the deposited sulfur-containing layer at potentials more negative than approximately −0.9 V, and formation of a sulfur-containing multilayer at potentials more positive than −0.2 V. During the reduction of sulfur deposited on Au, a mass increase due to the formation of polysulfide species in the surface layer, accompanied by incorporation of Na+ counterions, can be observed that starts at approximately −0.4 V. This is a reversible process, i.e., during the reoxidation, counterions leave the surface layers. Frequency excursions during the electroreduction and reoxidation processes reveal existence of several competitive dissolution–deposition steps. Spontaneous interaction between Au and HS− species results in a surface mass increase at the open-circuit potential, and it also manifests itself in the substantial decrease of the open-circuit potential after addition of Na2S to the supporting electrolyte.







Similar content being viewed by others
References
Buckley AN, Hamilton IC, Woods R (1987) J Electroanal Chem 216:213. doi:10.1016/0022-0728(87)80208-5
Lezna RO, de Tacconi NR, Arvia AJ (1990) J Electroanal Chem 283:319. doi:10.1016/0022-0728(90)87398-4
Gao X, Zhang Y, Weaver MJ (1992) Langmuir 8:668. doi:10.1021/la00038a060
Parker GK, Watling KM, Hope GA, Woods R (2008) Colloids Surfaces A 18:151. doi:10.1016/j.colsurfa.2007.12.029
Quijada C, Huerta FJ, Morallón E, Vázquez JL, Berlouis LEA (2000) Electrochim Acta 45:1847. doi:10.1016/S0013-4686(99)00396-5
Vericat C, Vela ME, Andreasen G, Salvarezza RC, Vazquez L, Martin-Gao JA (2001) Langmuir 17:4919. doi:10.1021/la0018179
Vericat C, Vela ME, Gago J, Salvarezza RC (2004) Electrochim Acta 49:3643. doi:10.1016/j.electacta.2004.02.046
Lay MD, Varazo K, Stickney JL (2003) Langmuir 19:8416. doi:10.1021/la034474y
Biener MM, Biener J, Friend CM (2005) Langmuir 21:1668. doi:10.1021/la047387u
De Tacconi NR, Rajeshwar K (1998) J Electroanal Chem 444:7. doi:10.1016/S0022-0728(97)00533-0
Myung N, Kim S, Lincot D, Lepiller C, de Tacconi NR, Rajeshwar K (2000) Electrochim Acta 45:3749. doi:10.1016/S0013-4686(00)00472-2
Licht S (1988) J Electrochem Soc 135:2971. doi:10.1149/1.2095471
Hepel M, Janusz W (2000) Electrochim Acta 45:3785. doi:10.1016/S0013-4686(00)00468-0
Zhdanov SI (1982) Sulfur. In: Bard AJ (ed) Encyclopedia of electrochemistry of elements, vol. 6. Marcel Dekker, New York, p 273
Zhdanov SI (1985) Sulfur, selenium, tellurium, and polonium. In: Bard AJ, Parsons R, Jordan J (eds) Standard potentials in aqueous solution. Marcel Dekker, New York, p 93
Levillain E, Demortier A, Lelieur J-P (2006) Sulfur. In: Scholz F, Pickett CJ (eds) Inorganic electrochemistry, Bard AJ, Stratmann M (eds) Encyclopedia of electrochemistry, vol 7a. Wiley-VCH, Weinheim, p 253
Orlik M, Galus Z (2006) Electrochemistry of gold. In: Scholz F, Pickett CJ (eds) Inorganic electrochemistry. Bard AJ, Stratmann M (eds) Encyclopedia of electrochemistry, vol 7b. Wiley-VCH, Weinheim, pp 853–854
Inzelt G (2006) Standard, formal, and other characteristic potential of selected electrode reactions. In: Scholz F, Pickett CJ (eds) Inorganic electrochemistry, Bard AJ, Stratmann M (eds) Encyclopedia of electrochemistry, vol 7a. Wiley-VCH, Weinheim, pp 64–66
Schmid GM (1985) Gold. In: Bard AJ, Parsons R, Jordan J (eds) Standard potentials in aqueous solution. Marcel Dekker, New York, p 313
Busev AI, Ivanov VI (1973) Analytical chemistry of gold. Nauka, Moscow, p 28 (in Russian)
Peschevskii BI, Anoshin TN, Ehrenburg AM (1965) Dokl AN USSR 162:915
Cherneva AN (1960) Trudi Uralskogo Polytech Inst 96:134
Morris T, Copeland H, Szulczewski G (2002) Langmuir 18:535. doi:10.1021/la011186y
Kotowski A (ed) (1954) Gmelins Handbuch der anorganische Chemie, vol 62 (Gold). Verlag Chemie, Weinheim, p 716
Bailar JC, Emeléus HJ, Nyholm R, Trotman-Dickensen AF (eds) (1973) Comprehensive inorganic chemistry, vol 3. Pergamon Press, Oxford New York, p 150
Inzelt G, Puskas Z, Nemeth K, Varga I (2005) J Solid State Electrochem 9:823. doi:10.1007/s10008-005-0019-5
Acknowledgment
This work is supported by bilateral program cooperation between Republic of Croatia and Hungary CRO-01/2006 as well as by national research projects no. 098-0982934-2717 from the Ministry of Science and Technology of the Republic of Croatia (IC and EB-N) and OTKA K71771 from the National Scientific Research Fund, Hungary (GI).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bura-Nakic, E., Róka, A., Ciglenecki, I. et al. Electrochemical nanogravimetric studies of sulfur/sulfide redox processes on gold surface. J Solid State Electrochem 13, 1935–1944 (2009). https://doi.org/10.1007/s10008-008-0742-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-008-0742-9