Abstract
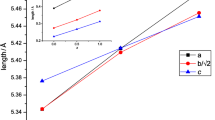
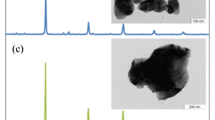
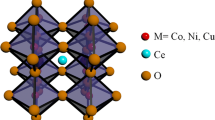
The electrochemical properties of Ca1 − x Ce x MnO3 perovskite-type oxide electrode have been investigated by cyclic voltammetry in Na2SO4 aqueous solutions with pH 14. The structural and morphological characterizations have also been investigated and the information used to interpret the electrochemical behavior. An estimation of the electrode’s capacitance and roughness factor has been obtained by means of cyclic voltammetry. The specific capacitance and consequently the roughness factor values are affected by the presence of Ce ions in the oxide. These findings are in agreement with the increase of the oxide-specific surface area by the introduction of Ce ion. The open-circuit potential and the voltammetric patterns are dependent on the presence of Ce ion in the electrodes and support that the surface electrochemistry of the perovskite oxide electrodes is governed by the Mn4+–Mn3+ redox couple.








Similar content being viewed by others
References
Bockris JO’M, Otagawa T (1984) J Electrochem Soc 131:290 doi:10.1149/1.2115565
Skinner SJ (2001) Int J Inorg Mater 3:113 doi:10.1016/S1466-6049(01)00004-6
Pereira MI, Melo MJBV, Costa FMA, Nunes MR, Peter LM (1989) J Chem Soc, Perkin Trans 1 85:2473
Hammouche A, Siebert E, Hammou A, Kleitz M (1991) J Electrochem Soc 138:1212 doi:10.1149/1.2085761
Morimoto H, Esaka T, Takai S (1997) Mater Res Bull 32:1359 doi:10.1016/S0025-5408(97)00113-X
Ciríaco MLF, da Silva Pereira MI, Nunes MR, Mendonça MH, Costa FM (2001) J Solid State Electrochem 5:495 doi:10.1007/s100080100227
Xiqiang H, Li P, Zhiguo L, Zhe L, Yu S, Zhengnan Q et al (2002) J Alloy Comp 345:265
Ciríaco MLF, da Silva Pereira MI, Nunes MR, Mendonça MH, Costa FM (2006) Mater Chem Phys 96:211 doi:10.1016/j.matchemphys.2005.07.005
Esaka T, Morimoto H, Iwahara H (1992) J Appl Electrochem 22:821 doi:10.1007/BF01023724
Iwahara H, Esaka T, Hamajina H (1989) Denki Kagaku 57:591
Melo Jorge ME, Nunes MR, Silva MR, Sousa D (2005) Chem Mater 17:2069 doi:10.1021/cm040188b
Singh KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquérol J et al (1985) Pure Appl Chem 57:603 doi:10.1351/pac198557040603
Rouquérol F, Rouquérol J, Sing KSW (1999) Adsorption by powders and porous solids. Academic, London
Ohtaki M, Koga H, Tokunaga T, Eguchi K, Arai H (1995) J Solid State Chem 120:105 doi:10.1006/jssc.1995.1384
Vecherskii SI, Konopel’ko MA, Esina NO, Batalov NN (2002) Inorg Mater 38:1270 doi:10.1023/A:1021379606219
Zeng Z, Greenblatt M, Croft M (2001) Phys Rev B 63:224410–224411 doi:10.1103/PhysRevB.63.224410
Melo Jorge ME, Correia dos Santos A, Nunes MR (2001) Int J Inorg Mater 3:915 doi:10.1016/S1466-6049(01)00088-5
Shannon RD (1976) Acta Crystallogr A 32:751 doi:10.1107/S0567739476001551
Klug H, Alexander L (1962) X-ray diffraction procedures. Wiley, New York
Trasatti S (1994) In: Lipkowski J, Ross PN (eds) Electrochemistry of novel materials. VCH, New York
Pourbaix M (1974) Atlas of electrochemical equilibria in aqueous solutions. NACE, Houston
Hayes SA, Yu P, O’Keefe TJ, O’Keefe MJ, Stoffer JO (2002) J Electrochem Soc 149:C623 doi:10.1149/1.1516775
Bockris JO’M, Khan SUM (1993) Surface electrochemistry, a molecular level approach. Plenum, New York
Chang C, Tsou T (2002) Electrochim Acta 47:3523 doi:10.1016/S0013-4686(02)00321-3
Levine S, Smith AL (1971) Discuss Faraday Soc 52:290 doi:10.1039/df9715200290
Wattiaux A, Grenier JC, Pouchard M, Hagenmuller P (1987) J Electrochem Soc 134:1714 doi:10.1149/1.2100741
Trasatti S, Kurzweil P (1994) Platinum Metal Rev 38:46
Mattos-Costa FI, Lima-Neto P, Machado SAS, Avaca LA (1998) Electrochim Acta 44:1515 doi:10.1016/S0013-4686(98)00275-8
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lucas, C., Eiroa, I., Nunes, M.R. et al. Preparation and characterization of Ca1 − x Ce x MnO3 perovskite electrodes. J Solid State Electrochem 13, 943–950 (2009). https://doi.org/10.1007/s10008-008-0630-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-008-0630-3