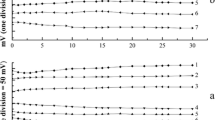
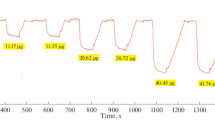
Abstract
Natural monocrystalline chalcopyrite and galena as new indicator electrodes for the potentiometric titration of weak acids in N,N-dimethylformamide and N-methylpyrrolidone were used. The investigated electrodes showed a linear dynamic response for p-toluenesulfonic acid concentrations in the range from 0.1 to 0.001 M, with a Nernstian slope of 59.0 mV for chalcopyrite and 33 mV per decade for galena in N,N-dimethylformamide, 56.1 mV for chalcopyrite, and 32.0 mV per decade for galena in N-methylpyrrolidone. The potential in the course of the titration and at the titration end point was rapidly established. Sodium methylate, potassium hydroxide, and tetrabutylammonium hydroxide proved to be very suitable titrating agents for these titrations. The response time was less than 10–11 s, and the lifetime of the electrodes is limitless. The advantages of the electrodes are log-term stability, fast response, reproducibility, easy preparation, and low cost. The results obtained in the determination of the investigated weak acids deviated on average by ±0.04–0.34% from those obtained with a glass electrode.







Similar content being viewed by others
References
Vyas RHQ, Kharat RB (1988) Indian J Pharm Sci 50(5):279
Fritz JS, Keen RT (1952) Anal Chem 24:308 doi:10.1021/ac60062a013
Rhodes HJ, DeNardo JJ, Bode DW, Blake MI (1975) J Pharm Sci 64:1386 doi:10.1002/jps.2600640828
Galpern GM, Kreshkov AP, Teplova VV, Sulpovar, Seryanova SE, Yanduganova NP (1977) Zh Anal Khim 32:586
Izutsu K (2002) Electrochemistry in nonaqueous solution. Wiley-VCS, Germany
Fauth MI, Frandsen M, Havlik BR (1964) Anal Chem 36:380 doi:10.1021/ac60208a041
Fritz JS, Keen RT (1953) Anal Chem 25:179 doi:10.1021/ac60073a040
Das A, Boparai KS (1982) Talanta 29:57 doi:10.1016/0039-9140(82)80138-0
Fritz JS (1952) Anal Chem 24:306 doi:10.1021/ac60062a012
Saad SM, Zaki TM (1975) Talanta 22:843 doi:10.1016/0039-9140(75)80180-9
Aslan A, Erdogan Y, Demirtas A, Karslioglu S (1997) Farmazie 52:309
Maurmeyer RK, Margosis M, Ma TS (1958) Mikrochim Acta 47:177 doi:10.1007/BF01218684
Deal VZ, Wyld GEA (1955) Anal Chem 27:47 doi:10.1021/ac60097a014
Ephraim JH (1989) Talanta 36:379 doi:10.1016/0039-9140(89)80204-8
Esteves da Silva JCG, Machado ASC (1994) Talanta 41:2095 doi:10.1016/0039-9140(94)00185-5
Butler JP, Czepiel TP (1956) Anal Chem 28:1468 doi:10.1021/ac60117a035
Kolthoff IM, Chantooni MK Jr, Smagowski H (1970) Anal Chem 42:1622 doi:10.1021/ac60295a013
Bartnicka H, Bojanowska I, Kalinowski MK (1991) Aust J Chem 44:1077
Mansfeld M, Pařik P, Ludwig M (2004) Collect Czechoslov Chem Commun 69:1479 doi:10.1135/cccc20041479
Alkan M, Yüksek H, Islamoğlu F, Bahçeci Ş, Calapoğlu M, Elmastaş M et al (2007) Molecules 12:1805
Bartnicka H, Bojanowska I, Kalinowski MK (1993) Aust J Chem 46:31
Barbosa J, Bosch CM, Sanz-Nebot V (1992) Mikrochim Acta 106:327 doi:10.1007/BF01242105
Barbosa J, Bosch CM (1991) Talanta 38:1297 doi:10.1016/0039-9140(91)80109-D
Roletto E, Vanni A (1977) Talanta 24:73 doi:10.1016/0039-9140(77)80195-1
Braun RD, LoVerso MR (1979) Talanta 26:185 doi:10.1016/0039-9140(79)80046-6
Galpern GM, Gurvich Ya A, Kryuchkova NF (1970) Zh Anal Khim 25:1819
Korenman YI, Ermolaeva TN, Podolina EA (1998) J Radioanal Nucl Chem 228:113 doi:10.1007/BF02387310
Korenman Ya I, Yermolaeva TN (1995) Analyst (Lond) 120:2387 doi:10.1039/an9952002387
Karlberg B, Johansson G (1969) Talanta 16:1545 doi:10.1016/0039-9140(69)80215-8
Bates RG (1973) Determination of pH, theory and practice. Wiley, New York, p 372
Mihajlović Lj V, Mihajlović RP, Antonijević MM, Vukanović BV (2004) Talanta 64:879 doi:10.1016/j.talanta.2004.03.061
Mihajlović R, Stanić Z (2005) J Solid State Electrochem 9:558 doi:10.1007/s10008-004-0591-0
Antonijević MM, Mihajlović RP, Vukanović BV, Jovanović S (1997) Analusis 25:152
Habashi F (1978) Chalcopyrite: its chemistry and metallurgy. McGraw-Hill, New York, p 165
Koch DFA (1975) In: Bockris JOM, Conway BE (eds) Modern aspects of electrochemistry. Plenum, New York, p 211
Folmer JCW, Jellinek F (1980) J Less-Common Met 76:153 doi:10.1016/0022-5088(80)90019-3
Nakai I, Sugitani Y, Nagashima K, Niwa Y (1978) J Inorg Nucl Chem 40:789 doi:10.1016/0022-1902(78)80152-3
Vaughan DJ, Graig JR (1978) Mineral chemistry of metal sulfides. Cambridge Earth Science Series. Cambridge University Press, New York, p 493
Pikna L, Lux L, Grygar T (2006) Chem Papers 60:293 doi:10.2478/s11696-006-0051-7
Hackl RP, Dreisinger DB, Peters E, King JP (1995) Hydrometallurgy 39:25 doi:10.1016/0304-386X(95)00023-A
Buckley AN, Woods R (1994) J Electroanal Chem 370:295 doi:10.1016/0022-0728(94)03211-4
Schuhmann D (1993) N J Chem 17:551
Ndzebet E, Schuhmann D, Vanel P (1994) Electrochim Acta 39:745 doi:10.1016/0013-4686(94)80019-7
Peuporte T, Schuhmann D (1995) J Electroanal Chem 385:9 doi:10.1016/0022-0728(94)03756-S
Nowak P, Laajalehto K, Kartio I (2000) Colloids Surf 161:447 doi:10.1016/S0927-7757(99)00214-9
Kim BS, Hayers RA, Prestige CA, Ralson J, St Smart R (1995) Langmuir 11:2554 doi:10.1021/la00007a039
Wittstock G, Kartio I, Hirsch D, Kunze S, Szargan R (1996) Langmuir 12:5709 doi:10.1021/la960385s
Lam-Thi PO, Lamache M, Bauer D (1984) Electrochim Acta 29:217 doi:10.1016/0013-4686(84)87050-4
Paul RL, Nicol ML, Diggle JW, Saunders AP (1979) Electrochim Acta 23:625 doi:10.1016/0013-4686(78)80091-7
Kuruoğlu D, Canel E, Memon S, Yilmaz M (2003) Anal Sci 19:217 doi:10.2116/analsci.19.217
Oyama N, Hirokawa T, Yamaguchi S, Ushizawa N, Shimomura T (1987) Anal Chem 59:258 doi:10.1021/ac00129a009
Acknowledgment
This work was funded by the Ministry of Science of the Republic of Serbia (project no. 142060 B)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mihajlović, L.V., Nikolić-Mandić, S.D., Vukanović, B.V. et al. Natural monocrystalline chalcopyrite and galena as electrochemical sensors in non-aqueous solvents. Part II: potentiometric titrations of weak acids in N,N-dimethyformamide and N-methylpyrrolidone. J Solid State Electrochem 13, 895–904 (2009). https://doi.org/10.1007/s10008-008-0625-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-008-0625-0