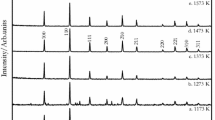
Abstract
The Gibbs free energies of formation of Eu3RuO7(s) and Eu2Ru2O7(s) have been determined using solid-state electrochemical technique employing oxide ion conducting electrolyte. The reversible electromotive force (e.m.f.) of the following solid-state electrochemical cells have been measured:
The Gibbs free energies of formation of Eu3RuO7(s) and Eu2Ru2O7(s) from elements in their standard state, calculated by the least squares regression analysis of the data obtained in the present study, can be given, respectively, by:
The uncertainty estimates for Δf G o(T) include the standard deviation in e.m.f. and uncertainty in the data taken from the literature.

Similar content being viewed by others
References
Cao G, McCall S, Crow JE, Guertin RP (1997) Phys Rev Lett 78:1751
Taira N, Wakeshima M, Hinatsu Y (1999) J Phys: Condens Matter 11:6983
van Berkel FPF, Ijdo DJW (1986) Mater Res Bull 21:1103
Harada D, Hinatsu Y (2001) J Phys: Condens Matter 13:10825
Harada D, Hinatsu Y (2001) J Solid State Chem 158:245
Taira N, Wakeshima M, Hinatsu Y (1999) J Solid State Chem 144:216
Taira N, Wakeshima M, Hinatsu Y, Tobo A, Ohoyama K (2003) J Solid State Chem 176:165
Pike GE, Seager CH (1977) J Appl Phys 48:5152
Carcia PF, Ferreti A, Suna A (1982) J Appl Phys 53:5282
Horowitz HS, Longo JM, Horowitz HH (1983) J Electrochem Soc 130:1851
Egdell RG, Goodenough JB, Hamnett A, Naish CC (1983) J Chem Soc Faraday Trans 79:893
Taira N, Wakeshima M, Hinatsu Y (2000) J Solid State Chem 152:441
Smith, McCarthy (1975) Penn State Univ., Univ. Park, Pennsylvania, U.S.A., JCPDS Grant-in-Aid Report
Cao G, McCall S, Zhou ZX, Alexander CS, Crow JE, Guertin RP, Mielke CH (2001) Phys Rev B 63:144427
Cao G, McCall S, Zhou ZX, Alexander CS, Crow JE, Guertin RP (2001) J Magn Magn Mater 226:218
Guertin RP, McCall S (2001) Inter J Mod Phys B 16:3317
Pratt JN (1990) Metall Trans A 21A:1223
Singh Z, Dash S, Prasad R, Sood DD (1994) J Alloys Compd 215:303
Barin I (1995) Thermochemical Data of Pure Substances, vols. I and II, third edn. VCH, New York
Pankratz LB (1982) Thermodynamic Properties of Elements and Oxides, Bulletin 672. United States Bureau of Mines
Acknowledgement
The authors are grateful to Dr. K. D. Singh Mudher for providing the X-ray diffraction analysis performed by his group.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Banerjee, A., Prasad, R. & Venugopal, V. Thermodynamic properties of the ternary oxides in the Eu–Ru–O system by using solid-state electrochemical cells. J Solid State Electrochem 11, 291–295 (2007). https://doi.org/10.1007/s10008-006-0106-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-006-0106-2