Abstract
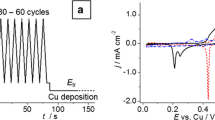
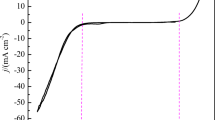
The early stages of Cu electrodeposition onto Pt(poly) have been investigated in 0.5 M H2SO4 + 0.01 M CuSO4 solution without or with H2SeO3 when a molar concentration ratio [Cu(II)]/[Se(IV)] ≥ 2×102 using electrochemical and ex situ AFM techniques. The overpotential deposition of Cu has been performed onto a Pt surface precovered independently with Cu in amount close to an equivalent monolayer. Chronoamperometric results have been shown to follow an instantaneous 3D nucleation and diffusion-controlled growth model. The values of diffusion coefficient D for Cu2+, number of nuclei N and average nuclei radius r av have been calculated. In the local regions of the surface, the separate large agglomerates composed of the different diameter clusters have been revealed in both cases, but, in the presence of the H2SeO3, they attained a distinct chain-like configuration. Some morphological characteristics have been reported.











Similar content being viewed by others
References
Budevski E, Staikov G, Lorenz WJ (2000) Electrochim Acta 45:2559
Lorenz WJ, Staikov G, Schindler W, Wiesbeck W (2002) J Electrochem Soc 149:K47
Martins ME, Salvarezza RC, Arvia AJ (1992) Electrochim Acta 37:2203
Kolb DM (1978) Physical and electrochemical properties of metal monolayers on metallic substrates. In: Gerischer H, Tobias ChW (eds) Advances in electrochemistry and electrochemical engineering, vol 11. Wiley, New York, pp 125–271
Furuya N, Motoo S (1976) J Electroanal Chem 72:165
Hammond JS, Winograd N (1977) J Electroanal Chem 80:123
Margheritis D, Salvarezza RC, Giordano MC, Arvia AJ (1987) J Electroanal Chem 229:327
Plieth W (1992) Electrochim Acta 37:2115
Nichols RJ, Beckmann W, Meyer H, Batina N, Kolb DM (1992) J Electroanal Chem 330:381
Michailova E, Vitanova I, Stoychev D, Milchev A (1993) Electrochim Acta 38:2455
Fabricius G, Kontturi K, Sundholm G (1994) Electrochim Acta 39:2353
Rynders RM, Alkire RC (1994) J Electrochem Soc 141:1166
Peykova M, Michailova E, Stoychev D, Milchev A (1995) Electrochim Acta 40:2595
Rashkov R, Nanev C (1995) J Appl Electrochem 25:603
Hölzle MH, Apsel CW, Will T, Kolb DM (1995) J Electrochem Soc 142:3741
Tarallo A, Heerman L (1999) J Appl Electrochem 29:585
Radisic A, West AC, Searson PC (2002) J Electrochem Soc 149:C94
Llorca MJ, Herrero E, Feliu JM, Aldaz A (1994) J Electroanal Chem 373:217
Herrero E, Rodes A, Pérez JM, Feliu JM, Aldaz A (1996) J Electroanal Chem 412:165
Bhattacharya RN, Fernandez AM, Contreras MA, Keane J, Tenant A, Ramanathan K, Tuttle JR, Noufi RN, Hermann AM (1996) J Electrochem Soc 143:854
Lippkow D, Strehblow H-H (1998) Electrochim Acta 43:2131
Carbonnelle P, Lamberts L (1992) J Electroanal Chem 340: 53
Massaccesi S, Sanchez S, Vedel J (1993) J Electrochem Soc 140:2540
Marlot A, Vedel J (1999) J Electrochem Soc 146:177
Kemell M, Saloniemi H, Ritala M, Leskelä M (2000) Electrochim Acta 45:3737
Hill MRH, Rogers GT (1976) J Electroanal Chem 68:149
Lezhava TI (1989) Acceleration at Metal Deposition. Diss Frumkin Inst Electrochem, Moscow
Steponavičius A, Šimkūnaitė D, Jasulaitienė V (1997) Chemija No 2:64
Riveros G, Henriquez R, Córdova R, Schrebler R, Dalchiele EA, Gómez H (2001) J Electroanal Chem 504:160
Steponavičius A, Šimkūnaitė D, Lichušina S, Kapočius V (2001) Chemija 12:147
Steponavičius A, Šimkūnaitė D (2002) Bull Electrochem 18:367
Biegler T, Rand DAJ, Woods R (1971) J Electroanal Chem 29:269
Steponavičius A, Šimkūnaitė D (2002) Rus J Electrochem 38:488
Jerkiewicz G, Vatankhab Gh, Lessard J, Soriaga MP, Park Y-S (2004) Electrochim Acta 49:1451
Danilov AI, Molodkina EB, Polukarov YuM (2000) Rus J Electrochem 36:976
Šimkūnaitė D, Ivaškevič E, Jasulaitienė V, Steponavičius A (2003) Bull Electrochem 19:437
Šimkūnaitė D, Ivaškevič E, Jasulaitienė V, Kaliničenko A, Valsiūnas I, Steponavičius A (2004) Chemija 15:12
Bosco E, Rangarajan SK (1981) J Chem Soc Faraday Trans 1 77:1673
Breiter MW (1969) Trans Faraday Soc 65:2197
Jüttner K, Lorenz WJ, Staikov G, Budevski E (1978) Electrochim Acta 23:741
Gunawardena GA, Hills GJ, Montenegro I (1978) Electrochim Acta 23:693
Gunawardena GA, Hills G, Montenegro I, Scharifker B (1982) J Electroanal Chem 138:225
Scharifker B, Hills G (1983) Electrochim Acta 28:879
Grujicic D, Pesic B (2002) Electrochim Acta 47:2901
Leone A, Marino W, Scharifker BR (1992) J Electrochem Soc 139:438
Quickenden TI, Xu Q (1996) J Electrochem Soc 143:1248
Yu J, Wang L, Su L, Ai X, Yang H (2003) J Electrochem Soc 150:C19
Palmisano F, Desimoni E, Sabbatini L, Torsi G (1979) J Appl Electrochem 9:517
Ehlers C, König U, Staikov G, Schultze JW (2001) Electrochim Acta 47:379
Serruya A, Mostany J, Scharifker BR (1999) J Electroanal Chem 464:39
Radisic A, Long JG, Hoffmann PM, Searson PC (2001) J Electrochem Soc 148:C41
Danilov AI, Molodkina EB, Baitov AA, Pobelov IV, Polukarov YuM (2002) Rus J Electrochem 38:743
Oskam G, Searson PC (2000) J Electrochem Soc 147:2199
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Šimkūnaitė, D., Ivaškevič, E., Kaliničenko, A. et al. Nucleation and growth of Cu onto polycrystalline Pt electrode from acidic CuSO4 solution in the presence of H2SeO3 . J Solid State Electrochem 10, 447–457 (2006). https://doi.org/10.1007/s10008-005-0034-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-005-0034-6