Abstract
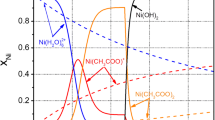
Electrochemical deposition of a two-component Ni-Cu coating onto Fe powder particles was studied by constant current electrolysis. The influence of the Ni(II) to Cu(II) salts ratio in the electrolyte, of the addition of sodium citrate as complexing agent to the electrolyte, and of the current density with respect to the total surface of compact and dispersed electrodes was studied. It was shown that the less noble Ni deposition is facilitated mainly by its large excess in the electrolyte and by an increase in current density. The addition of a complexing agent exhibits only a very slight influence on the coating composition but does suppress the spontaneous deposition of Cu. The current efficiencies of Ni, Cu and Ni-Cu deposition on the powder particles decrease with increasing sodium citrate concentration and current density.





Similar content being viewed by others
References
Levy EM (1969) Plating 56:903
Venkatsetty HV (1970) J Electrochem Soc 117:403
Tutovan V, Velican N (1971) Thin Solid Films 7:219
Morral FR (1972) Plating 59:131
Muntenau A, Schiff A, Marcu C (1970) Chem Abstr 73:50125x
Racinskiene S, Khotyanovich SI (1974) Chem Abstr 81:71781l
Lux L, Gálová M, Oriňáková R, Turoňová A (1999) Particulate Sci Technol 16:135
Gálová M, Lux L, Dudrová E, Stašková R (1994) Trans Techn Univ Košice 3/4:185
Fleischmann M, Oldfield JW (1971) J Electroanal Chem 29:211
Losev AV, Petrij OA (1975) Elektrokhimiya 11:1243
Held J, Gerischer H (1963) Ber Bunsenges Phys Chem 67:921
Hurwitz HD, Srinivasan S, Litt M (1971) Ber Bunsenges Phys Chem 75:535
Sabacky BJ, Evans JW (1977) Metall Trans 8B:5
Lux L, Stašková R, Gálová M (1996) Acta Chim Models Chem 133:115
Gálová M, Lux L, Oriňáková R (1998) J Solid State Electrochem 2:2
Green TA, Russel AE, Roy S (1998) J Electrochem Soc 145:3
Brenner A (1969) Electrodeposition of alloys. Academic Press, New York
Schlesinger M, Paunovic M (2000) Modern electroplating. Wiley, New York
Daniele PG, Ostacoli G, Rigano C, Sammartano S (1984) Transition Met Chem 9:385
Scholz F, Lange B (1992) Trends Anal Chem 11:360
Acknowledgements
Financial support of the Grant Agency of the Slovak Republic within the frames of project no. 1/9038/02 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Turoňová, A., Gálová, M. & Šupicová, M. Parameters influencing the electrodeposition of a Ni-Cu coating on Fe powders. I. Effect of the electrolyte composition and current density. J Solid State Electrochem 7, 684–688 (2003). https://doi.org/10.1007/s10008-003-0372-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-003-0372-1