Abstract.
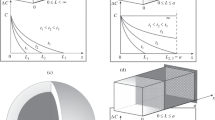
The kinetics of lithium transport through a Carbotron-P hard carbon composite electrode was investigated by analysis of the current transients based upon the modified McNabb-Foster equation. The electrode potential curve ran continuously throughout the whole deintercalation of lithium ions, without the appearance of any potential plateau. However, the anodic current transients showed inflexion points, indicating that several kinds of lithium deintercalation sites present within the electrode are kinetically distinguishable among themselves. Moreover, the anodic current transients did not follow Cottrell behaviour but Ohmic behaviour. The anodic current transients experimentally measured coincided well with those transients numerically simulated based upon the modified McNabb-Foster equation as a governing equation and the "cell-impedance-controlled" constraint as a boundary condition. This strongly indicates that lithium transport is governed by "cell impedance" and at the same time the difference in activation energies for lithium deintercalation between the four different lithium deintercalation sites existing within the electrode accounts for the different kinetics of lithium transport between these sites.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Chang, WY., Pyun, SI. & Lee, SB. Kinetics of lithium transport through a hard carbon electrode studied by analysis of current transients. J Solid State Electrochem 7, 368–373 (2003). https://doi.org/10.1007/s10008-002-0329-9
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10008-002-0329-9