Abstract.
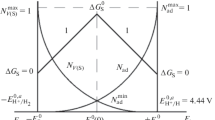
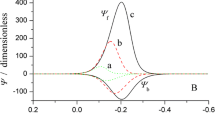
Two types of non-integer electron-exchange numbers from uniform and reversible surface-redox reactions without side reactions have been distinguished. The first being the apparent number, n app, of the apparent faradaic charge corresponding to cyclovoltammetric peak areas above the interpolated baseline, and the second the thermodynamically defined surface-redox valency, n', of Nernstian slopes of cyclovoltammetric peak potentials depending on different solution pH. An analytical expression has been derived for n app based on a simplified capacitive equivalent circuit and for n' using the potential-dependent free adsorption energies of the reactants involved. It should be pointed out that the different experimental values of n app and n' refer to the same integer number of electrons per molecule oxidized or reduced.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Huck, H. The non-integer cyclovoltammetric electron-exchange numbers of reversible redox reactions of adsorbates: theoretical considerations. J Solid State Electrochem 6, 534–539 (2002). https://doi.org/10.1007/s10008-002-0279-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10008-002-0279-2