Abstract.
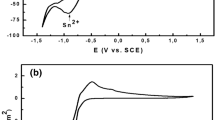
The mechanism of alkaline earth metal tungstate formation during soft solution processing was studied by cyclic voltammetry, electrochemical impedance spectroscopy and by direct in situ observation of the surface changes using atomic force microscopy. The electrochemical oxidation of W to WO3 was followed by dissolution of WO3 and, with some delay, by precipitation of tungstates at the metal surface. The same Tafel slopes observed in Li+, Ba2+, Sr2+ and Ca2+ containing solutions indicate that the course of the oxidation process is independent of the cation present in solution. The observed differences in the current-voltage curves outside the Tafel region are accounted for by the different film-forming tendencies of the various alkaline earth metal cations. The growth of tungstate layers at the W substrate decreases the electrochemically active area and limits the production of WO4 2– at later stages of deposition. At low potentials (E<0.2 V) the oxidation of W is the rate-controlling step. At higher potentials, however, the dissolution process slows down due to a relative decrease of the pH in the electrode vicinity and dissolution becomes the rate-limiting step.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Krtil, P., Fattakhova, D. & Yoshimura, M. Mechanism of soft solution processing formation of alkaline earth metal tungstates: an electrochemical and in situ AFM study. J Solid State Electrochem 6, 367–373 (2002). https://doi.org/10.1007/s10008-001-0260-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10008-001-0260-5