Abstract
Background
The present study investigated the expression of COX-2, EMMPRIN, HIF-1α, and GLUT-1 in the gingival tissue to verify if there is a correlation between the immunoexpression of these proteins and the changes caused by the inflamed infiltrate present in the gingival tissues.
Material and methods
A morphological analysis of epithelial changes (hyperplasia, exocytosis, spongiosis, and hydropic degeneration) was performed, as well as a semiquantitative analysis of the immunoexpression of COX-2, EMMPRIN, HIF-1α, and GLUT-1 in the epithelium and connective tissue of 60 specimens of gingival tissue.
Results
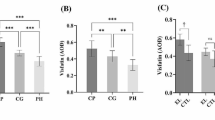
Epithelial immunoexpression to COX-2 was observed in three cases, while EMMPRIN, HIF-1α, and GLUT-1 were strongly expressed in the basal layer of the epithelium and gradually decreased until the upper layers. In the connective tissue, COX-2 immunoexpression showed a statistically significant association (p < 0.001) with the gingival inflammatory infiltrate. In connective tissue, EMMPRIN and HIF-1α exhibited intense immunopositivity, while GLUT-1 was negative in most cases.
Conclusion
COX-2 expression may constitute a biological marker of gingival tissues since its epithelial immunoexpression may indicate a greater propensity for the establishment of periodontal disease.


Similar content being viewed by others
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Holmstrup P, Plemons J, Meyle J (2018) Non–plaque-induced gingival disease. J Periodontol 89:S28–S45
Kumar S (2019) Evidence-based update on diagnosis and management of gingivitis and periodontitis. Dent Clin North Am 63:69–81
Lazăr L, Loghin A, Bud E, Cerghizan D, Horváth E, Nagy EE (2015) Cyclooxygenase-2 and matrix metalloproteinase-9 expressions correlate with tissue inflammation degree in periodontal disease. Rom J Morphol Embryol 56:1441–1446
Arweiler NB, Netuschil L (2016) The oral microbiota. Adv Exp Med Biol 902:45–60
Stone SJ, Kumar PS, Offenbacher S, Heasman PA, McCracken GI (2019) Exploring a temporal relationship between biofilm microbiota and inflammatory mediators during resolution of naturally-occurring gingivitis. J Periodontol 90:627–636
de Oliveira Nóbrega FJ, de Oliveira DHIP, Vasconcelos RG, Nonaka CFW, Queiroz LMG (2016) Study of the participation of MMP-7, EMMPRIN and cyclophilin A in the pathogenesis of periodontal disease. Arch Oral Biol 72:172–178
Liu X, Zhang Z, Pan S, Shang S, Li C (2018) Interaction between the Wnt/β-catenin signaling pathway and the EMMPRIN/MMP-2, 9 route in periodontitis. J Periodontal Res 53:842–852
Zhang Z, Yang X, Zhang H, Liu X, Pan S, Li C (2018) The role of extracellular matrix metalloproteinase inducer glycosylation in regulating matrix metalloproteinases in periodontitis. J Periodontal Res 53:391–402
Zhu P, Ding J, Zhou J, Dong W-J, Fan C-M, Chen Z-N (2005) Expression of CD147 on monocytes/macrophages in rheumatoid arthritis: its potential role in monocyte accumulation and matrix metalloproteinase production. Arthritis Res Ther 7:R1023–R1033
Huang N, Gibson FC III (2014) Immuno-pathogenesis of periodontal disease: current and emerging paradigms. Current Oral Health Report 1:124–132
Afacan B, Öztürk VO, Paşalı Ç, Bozkurt E, Köse T, Emingil G (2019) Gingival crevicular fluid and salivary HIF-1α, VEGF, and TNF-α levels in periodontal health and disease. J Periodontol 90:788–797
Liu L, Li C, Cai X, Xiang J, Cao Z, Dong W (2010) The temporal expression and localization of extracellular matrix metalloproteinase inducer (EMMPRIN) during the development of periodontitis in an animal model. J Periodontal Res 45:541–549
Morton RS, Dongari-Bagtzoglou AI (2001) Cyclooxygenase-2 is upregulated in inflamed gingival tissues. J Periodontol 72:461–469
Xiang J, Cao Z, Dong W, Li C (2009) Expression of extracellular matrix metalloproteinase inducer (EMMPRIN) in healthy and inflamed human gingival. Quintessence Int 44:125–132
Ng KT, Li JP, Ng KM, Tipoe GL, Leung WK, Fung ML (2011) Expression of hypoxia-inducible factor-1α in human periodontal tissue. J Periodontol 82:136–141
Ito H, Duxbury M, Zinner MJ, Ashley SW, Whang EE (2004) Glucose transporter-1 gene expression is associated with pancreatic cancer invasiveness and MMP-2 activity. Surgery 136:548–556
Idrees MM, Azzeghaiby SN, Hammad MM, Kujan OB (2014) Prevalence and severity of plaque-induced gingivitis in a Saudi adult population. Saudi Med J 25:1373–1377
Soanca A, Lupse M, Moldovan M et al (2018) Application of inflammation-derived gingival stem cells for testing the biocompatibility of dental restorative biomaterials. Annals Anat – Anat Anz 218:28–39
Magán-Fernández A, O’Valle F, Abadía-Molina F, Muñoz R (2019) Puga-Guil P and Mesa F Characterization and comparison of neutrophil extracellular traps in gingival samples of periodontitis and gingivitis: a pilot study. J Periodontal Res 54:218–224
Bage T, Lindeberg J, Lundeberg J, Modeer T, Yucel-Lindeberg T (2010) Signal pathways JNK and NF-κB, identified by global gene expression profiling, are involved in regulation of TNFα-induced mPGES-1 and COX- 2 expression in gingival fibroblasts. BMC Genomics 11:241–268
Bage T, Kats A, Lopez BS et al (2011) Expression of prostaglandin e synthases in periodontitis immunolocalization and cellular regulation. Am J Pathol 178:1676–1688
Mesa F, O’Valle F, Rizzo M et al (2014) Association between COX-2 rs 6681231 genotype and Interleukin-6 in periodontal connective tissue. A pilot study. Plos One 9:e87023
Moro MG, Oliveira MDS, Oliveira LR et al (2019) Effects of selective versus non-selective COX-2 inhibition on experimental periodontitis. Braz Dent J 30:133–138
Xue L, Su L, Xie J, Du Y, Yu X (2018) EMMPRIN-Cypa contributes to the inflammatory process in human periodontitis through infiltrating CD68+ inflammatory cells. Int J Clin Exp Pathol 11:3828–3832
Iacono KT, Brown A, Greene MI, Saouaf SJ (2007) CD147 Immunoglobulin superfamily receptor function and role in pathology. Exp Mol Pathol 83:283–295
Agrawal SM, Yong VW (2011) The many faces of EMMPRIN - roles in neuroinflammation. Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease 1812:213–219
Koinsky D, Campbell EL, Colgan SP (2010) Metabolic shift in immunity and inflammation. J Immunol 184:4062–4069
Imtyaz HZ, Simon MC (2010) Hypoxia-inducible factors as essential regulators of inflammation. Curr Top Microbiol Immunol 345:105–120
Vasconcelos RC, Costa ALL, Freitas RA et al (2016) Immunoexpression of HIF-1α and VEGF in periodontal disease and healthy gingival tissues. Braz Dent J 27:117–122
Yan K, Lin O, Tang K et al (2020) Substance P participates in periodontitis by upregulating HIF-1 α and RANKL/OPG ratio. BMC Oral Health 20:27
Angadi VC, Angadi PV (2015) GLUT-1 immunoexpression in oral epithelial dysplasia, oral squamous cell carcinoma, and verrucous carcinoma. J Oral Sci 57:115–122
Acknowledgements
We thank the Pathological Anatomy Service of the Discipline of Oral Pathology, Department of Dentistry, Federal University of Rio Grande do Norte, for providing the material and laboratory resources necessary for the study. This study was supported by the National Council for Scientific and Technological Development (CNPq, Brazil), the Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil), and the Postgraduate Program in Dental Sciences (UFRN, Natal, RN, Brazil).
Author information
Authors and Affiliations
Contributions
Déborah Pitta-Paraíso Iglesias: Participation in conducting the research and preparation of the manuscript. Weslay Rodrigues da Silva: Participation in conducting the research and preparation of the manuscript. Glória Maria de França and Caio César da Silva Barros: Wrote the main manuscript text. Roseana de Almeida Freitas and Hébel-Cavalcanti Galvão: Prepared figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All procedures performed in this retrospective study involving human participants were approved by the Institutional Ethics Committee, Protocol number 164/2012, C.A.A.E. 0279.0.051.051–11 (UFRN).
Consent to participate
All patients involved in this study signed the informed consent form.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iglesias, DPP., da-Silva, WR., de-França, GM. et al. Biological marker for the establishment of periodontal disease: cross-sectional study in the gingival tissue. Oral Maxillofac Surg 28, 217–223 (2024). https://doi.org/10.1007/s10006-022-01131-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10006-022-01131-5