Abstract
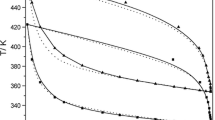
Thermal analysis has been integrated into our physical chemistry laboratory course in order to allow students to systematically explore the role of composition in determining the physical properties of a binary mixture. The β-naphthol/acetamide system is presented as an interesting starting point for such a project, as it displays a temperature/composition phase diagram which deviates slightly from the simple two-component eutectic picture. Students compare their measured pure-component melting temperatures with literature values, rationalize the observed trends with mixing using freezing-point-depression arguments, and make a literature search of the unique morphological solid phase obseved in analogous studies. This hands-on project is designed to be accompanied by a classroom discussion of multicomponent phase equilibria.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
KIM, A., MUSFELDT, J.L. Thermodynamics of the β-Naphthol/Acetamide Phase Diagram by Thermal Analysis Techniques. Chem. Educator 2, 1–10 (1997). https://doi.org/10.1007/s00897970129a
Issue Date:
DOI: https://doi.org/10.1007/s00897970129a