Abstract
Context
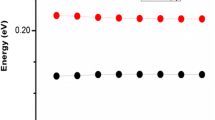
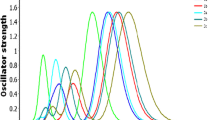
This study delves into hole-electron transport and distribution properties inherent in mono-brominated dibenzofuran (DBF) and dibenzothiophene (DBT) isomers. As determined by frontier molecular orbitals, all brominated structures have narrower bandgaps than their primary structures. The TD-DFT calculation showed that 2BDBT had the highest absorption wavelength of all molecules at 315.35 nm. Notably, the study unveils remarkably low electron and hole reorganization energies due to bromine substitution in DBF and DBT molecules. Specifically, the 4BDBF has the lowest hole reorganization energy of all DBF configurations, 0.229 eV. In addition, 3BDBF has 0.226 eV less electron reorganization energy than all other molecules. Compared to DBT, 3BDBT has the lowest electron reorganization energy of 0.254 eV. Overall, this research sheds significant light on the fundamental electronic and hole transport characteristics of bromine-substituted DBF and DBT isomers, highlighting their promising role in polymer design as donors/acceptors for advanced organic electronic applications.
Methods
Molecular structures were optimized using Density Functional Theory (DFT) B3LYP/6-311 + + G (d, p) level of theory, and the study further elucidates these molecules’ energy levels and absorption spectra through Time-Dependent Density Functional Theory TD-DFT; these calculations were performed using Gaussian 09W software package. The key parameters such as reorganization energies, Electron Localization Function map, Laplacian Bond Order, and NCI-RDG were meticulously examined for the molecules with the results of DFT calculations were analyzed and displayed by utilizing the software packages VMD 1.9.4 and Multiwfn 3.8, aiming to comprehend their charge transport and distribution properties.









Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Bronstein H, Nielsen CB, Schroeder BC, McCulloch I (2020) The role of chemical design in the performance of organic semiconductors. Nat Rev Chem 4:66–77. https://doi.org/10.1038/s41570-019-0152-9
Fahlman M, Fabiano S, Gueskine V, Simon D, Berggren M, Crispin X (2019) Interfaces in organic electronics. Nat Rev Mater 4:627–650. https://doi.org/10.1038/s41578-019-0127-y
Chen F-C (2018) Organic semiconductors, in: Encyclopedia of modern optics, Elsevier pp. 220–231. https://doi.org/10.1016/B978-0-12-803581-8.09538-2
Scholz R (2005) Organic semiconductors. In: Encyclopedia of condensed matter physics, Elsevier, pp 206–221. https://doi.org/10.1016/B0-12-369401-9/00658-6
Mortimer RJ, Dyer AL, Reynolds JR (2006) Electrochromic organic and polymeric materials for display applications. Displays 27:2–18. https://doi.org/10.1016/j.displa.2005.03.003
Zhao X, Chaudhry ST, Mei J (2017) Heterocyclic building blocks for organic semiconductors, pp 133–171. https://doi.org/10.1016/bs.aihch.2016.04.009
Zhang H, Su Q, Chen S (2020) Quantum-dot and organic hybrid tandem light-emitting diodes with multi-functionality of full-color-tunability and white-light-emission. Nat Commun 11:2826. https://doi.org/10.1038/s41467-020-16659-x
Kelley TW, Baude PF, Gerlach C, Ender DE, Muyres D, Haase MA, Vogel DE, Theiss SD (2004) Recent progress in organic electronics: materials, devices, and processes. Chem Mater 16:4413–4422. https://doi.org/10.1021/cm049614j
Yan C-C, Wang X-D, Liao L-S (2020) Organic lasers harnessing excited state intramolecular proton transfer process. ACS Photonics 7:1355–1366. https://doi.org/10.1021/acsphotonics.0c00407
Lee MY, Lee HR, Park CH, Han SG, Oh JH (2018) Organic transistor-based chemical sensors for wearable bioelectronics. Acc Chem Res 51:2829–2838. https://doi.org/10.1021/acs.accounts.8b00465
Abdurahman A, Hele TJH, Gu Q, Zhang J, Peng Q, Zhang M, Friend RH, Li F, Evans EW (2020) Understanding the luminescent nature of organic radicals for efficient doublet emitters and pure-red light-emitting diodes. Nat Mater 19:1224–1229. https://doi.org/10.1038/s41563-020-0705-9
Sun B, Zhu C-H, Liu Y, Wang C, Wan L-J, Wang D (2017) Oriented covalent organic framework film on graphene for robust ambipolar vertical organic field-effect transistor. Chem Mater 29:4367–4374. https://doi.org/10.1021/acs.chemmater.7b00800
Naeem N, Shehzad RA, Ans M, Akhter MS, Iqbal J (2022) Dopant free triphenylamine‐based hole transport materials with excellent photovoltaic properties for high‐performance perovskite solar cells. Energy Technol 10. https://doi.org/10.1002/ente.202100838
Yin J, Hu Y, Ju X-H (2014) Theoretical investigations on charge-transfer properties of pentacenequinone derivatives as n-type organic semiconductors. J Mol Model 20:2460. https://doi.org/10.1007/s00894-014-2460-9
Zubair I, Khera RA, Naveed A, Shehzad RA, Iqbal J (2022) Designing the optoelectronic properties of BODIPY and their photovoltaic applications for high performance of organic solar cells by using computational approach. Mater Sci Semicond Process 148:106812. https://doi.org/10.1016/j.mssp.2022.106812
Maiti B, Wang K, Bunge SD, Twieg RJ, Dunietz BD (2020) Enhancing charge mobilities in self-assembled N⋯I halogen bonded organic semiconductors: a design approach based on experimental and computational perspectives. Org Electron 79:105637. https://doi.org/10.1016/j.orgel.2020.105637
Luo Z, Ma R, Chen Z, Xiao Y, Zhang G, Liu T, Sun R, Zhan Q, Zou Y, Zhong C, Chen Y, Sun H, Chai G, Chen K, Guo X, Min J, Lu X, Yang C, Yan H (2020) Altering the positions of chlorine and bromine substitution on the end group enables high‐performance acceptor and efficient organic solar cells. Adv Energy Mater 10. https://doi.org/10.1002/aenm.202002649
Tang ML, Bao Z (2011) Halogenated materials as organic semiconductors. Chem Mater 23:446–455. https://doi.org/10.1021/cm102182x
Xiao L, Huang G, Zhang H, Zhang X, Li Y, Li S, Jiang T, Han B, Zhang Y, Zhou H (2022) Light managements and transparent electrodes for semitransparent organic and perovskite solar cells. Solar RRL 6. https://doi.org/10.1002/solr.202100818
Wu H, Ma Z, Li M, Lu H, Tang A, Zhou E, Wen J, Sun Y, Tress W, Olsen JMH, Meloni S, Bo Z, Tang Z (2023) Impact of donor halogenation on reorganization energies and voltage losses in bulk-heterojunction solar cells. Energy Environ Sci 16:1277–1290. https://doi.org/10.1039/D3EE00174A
Etabti H, Fitri A, Benjelloun AT, Benzakour M, Mcharfi M (2024) Advancing optoelectronic performance of organic and perovskite photovoltaics: computational modeling of hole transport material based on end-capped dibenzocarbazole molecules. Res Chem Intermed 50:1895–1927. https://doi.org/10.1007/s11164-024-05244-2
Etabti H, Fitri A, Benjelloun AT, Benzakour M, Mcharfi M (2023) Designing and Theoretical Study of Dibenzocarbazole Derivatives Based Hole Transport Materials: Application for Perovskite Solar Cells. J Fluoresc 33:1201–1216. https://doi.org/10.1007/s10895-023-03144-z
Luo Y, Yao L, Gu W, Xiao C, Liao H, Ravva MK, Wang Y, Li Z, Zhang L, Lv A, Yue W (2020) Effect of halogenated substituent on the properties of aza-octacenes. Org Electron 85:105895. https://doi.org/10.1016/j.orgel.2020.105895
Wang H, Liu T, Zhou J, Mo D, Han L, Lai H, Chen H, Zheng N, Zhu Y, Xie Z, He F (2020) Bromination: an alternative strategy for non‐fullerene small molecule acceptors. Adv Sci 7. https://doi.org/10.1002/advs.201903784
Yang F, Li C, Lai W, Zhang A, Huang H, Li W (2017) Halogenated conjugated molecules for ambipolar field-effect transistors and non-fullerene organic solar cells. Mater Chem Front 1:1389–1395. https://doi.org/10.1039/C7QM00025A
Wang H, Han L, Zhou J, Liu T, Mo D, Chen H, Lai H, Zheng N, Xie Z, Zheng W, He F (2021) Isomerism: minor changes in the bromine substituent positioning lead to notable differences in photovoltaic performance. CCS Chemistry 3:2591–2601. https://doi.org/10.31635/ccschem.020.202000540
Ji Ram V, Sethi A, Nath M, Pratap R (2019) Five-membered heterocycles. In: The chemistry of heterocycles, Elsevier, pp 149–478. https://doi.org/10.1016/B978-0-08-101033-4.00005-X
Kwiecień H, Wodnicka A (2020) Five-membered ring systems: furans and benzofurans, pp 281–323. https://doi.org/10.1016/B978-0-12-819962-6.00007-5
Gilman H, Jacoby AL (1938) Dibenzothiophene: orientation and derivatives. J Org Chem 03:108–119. https://doi.org/10.1021/jo01219a003
Turkoglu G, Cinar ME, Ozturk T (2017) Thiophene-based organic semiconductors. Top Curr Chem 375:84. https://doi.org/10.1007/s41061-017-0174-z
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision C.01, Gaussian, Inc., Wallingford CT, 2010. http://www.gaussian.com/
Becke AD (1993) A new mixing of Hartree-Fock and local density-functional theories. J Chem Phys 98:1372–1377. https://doi.org/10.1063/1.464304
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Humphrey W, Dalke A, Schulten K (1996) VMD: Visual molecular dynamics. J Mol Graph 14:33–38. https://doi.org/10.1016/0263-7855(96)00018-5
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Marcus RA, Sutin N (1985) Electron transfers in chemistry and biology. Biochimica et Biophysica Acta (BBA)—Reviews on Bioenergetics 811:265–322. https://doi.org/10.1016/0304-4173(85)90014-X
Marcus RA (1993) Electron transfer reactions in chemistry. Theory and experiment. Rev Mod Phys 65:599–610. https://doi.org/10.1103/RevModPhys.65.599
Marcus RA (1965) On the theory of electron-transfer reactions. VI. Unified treatment for homogeneous and electrode reactions. J Chem Phys 43:679–701. https://doi.org/10.1063/1.1696792
McMahon DP, Troisi A (2010) Evaluation of the external reorganization energy of polyacenes. J Phys Chem Lett 1:941–946. https://doi.org/10.1021/jz1001049
Lu T, Chen F (2013) Bond order analysis based on the Laplacian of electron density in fuzzy overlap space. J Phys Chem A 117:3100–3108. https://doi.org/10.1021/jp4010345
Fleming I (1976) Frontier orbitals and organic chemical reactions. Wiley
Taouali W, Alimi K, SindhooNangraj A, Casida ME (2023) Density-functional theory (DFT) and time-dependent DFT study of the chemical and physical origins of key photoproperties of end-group derivatives of a nonfullerene acceptor molecule for bulk heterojunction organic solar cells. J Comput Chem 44:2130–2148. https://doi.org/10.1002/jcc.27186
Politzer P, Abu-Awwad F (1998) A comparative analysis of Hartree-Fock and Kohn-Sham orbital energies. Theor Chem Accounts: Theor Comput Model (Theoretica Chimica Acta) 99:83–87. https://doi.org/10.1007/s002140050307
Wolinski K, Hinton JF, Pulay P (1990) Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J Am Chem Soc 112:8251–8260. https://doi.org/10.1021/ja00179a005
Deepakvijay K, Prakasam A, Arivazhagan R, Anbarasan PM (2023) Insights into the structural, electronic, quantum chemical properties and molecular docking studies on novel NAMPT inhibitor molecule. Chem Phys Impact 7:100395. https://doi.org/10.1016/j.chphi.2023.100395
Lewis DFV, Ioannides C, Parke DV (1994) Interaction of a series of nitriles with the alcohol-inducible isoform of P450: computer analysis of structure—activity relationships. Xenobiotica 24:401–408. https://doi.org/10.3109/00498259409043243
Aihara J (1999) Reduced HOMO−LUMO gap as an index of kinetic stability for polycyclic aromatic hydrocarbons. J Phys Chem A 103:7487–7495. https://doi.org/10.1021/jp990092i
O’boyle NM, Tenderholt AL, Langner KM (2008) cclib: a library for package-independent computational chemistry algorithms. J Comput Chem 29:839–845. https://doi.org/10.1002/jcc.20823
Savin A, Nesper R, Wengert S, Fässler TF (1997) ELF: the electron localization function angew chem. Int Ed Engl 36:1808–1832. https://doi.org/10.1002/anie.199718081
Becke AD, Edgecombe KE (1990) A simple measure of electron localization in atomic and molecular systems. J Chem Phys 92:5397–5403. https://doi.org/10.1063/1.458517
Fuster F, Sevin A, Silvi B (2000) Topological analysis of the electron localization function (ELF) applied to the electrophilic aromatic substitution. J Phys Chem A 104:852–858. https://doi.org/10.1021/jp992783k
Pilmé J, Piquemal J-P (2008) Advancing beyond charge analysis using the electronic localization function: chemically intuitive distribution of electrostatic moments. J Comput Chem 29:1440–1449. https://doi.org/10.1002/jcc.20904
Hernández-Trujillo J, García-Cruz I, Martínez-Magadán JM (2005) Topological analysis of the electron density and of the electron localization function of pyrene and its radicals. Chem Phys 308:181–192. https://doi.org/10.1016/j.chemphys.2004.08.010
Johnson ER, Keinan S, Mori-Sánchez P, Contreras-García J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc 132:6498–6506. https://doi.org/10.1021/ja100936w
Saleh G, Gatti C, Lo Presti L, Contreras-García J (2012) Revealing non-covalent interactions in molecular crystals through their experimental electron densities. Chem A European J 18:15523–15536. https://doi.org/10.1002/chem.201201290
Contreras-García J, Johnson ER, Keinan S, Chaudret R, Piquemal J-P, Beratan DN, Yang W (2011) NCIPLOT: a program for plotting noncovalent interaction regions. J Chem Theory Comput 7:625–632. https://doi.org/10.1021/ct100641a
Ltayef M, Mbarek M, Almoneef MM, Alimi K (2023) Modeling of the photophysical and photovoltaic properties of an active layer based on the organic composite poly(2‐methoxy‐5‐(2‐ethyl‐hexyloxy)‐1,4‐phenylene‐vinylene) ( <scp>MEH‐PPV</scp> )–poly(3‐hexylthiophene) ( <scp>P3HT</scp> ): (6,6)‐phenyl <scp>C61</scp> butyric acid methyl ester ( <scp>PCBM</scp> ). Int J Quantum Chem 123. https://doi.org/10.1002/qua.27204
Xie L, Xiao N, Li L, Xie X, Li Y (2020) Theoretical insight into the interaction between chloramphenicol and functional monomer (methacrylic acid) in molecularly imprinted polymers. Int J Mol Sci 21:4139. https://doi.org/10.3390/ijms21114139
Pei L, Li D-Z, Zhang L-J (2019) Theoretical insights into the hydrogen bonding interaction in the complexation of epinephrine with uracil. J Mol Model 25:252. https://doi.org/10.1007/s00894-019-4123-3
Wu P, Chaudret R, Hu X, Yang W (2013) Noncovalent interaction analysis in fluctuating environments. J Chem Theory Comput 9:2226–2234. https://doi.org/10.1021/ct4001087
Cheung DL, Troisi A (2010) Theoretical study of the organic photovoltaic electron acceptor PCBM: morphology, electronic structure, and charge localization. J Phys Chem C 114:20479–20488. https://doi.org/10.1021/jp1049167
Malagoli M, Brédas JL (2000) Density functional theory study of the geometric structure and energetics of triphenylamine-based hole-transporting molecules. Chem Phys Lett 327:13–17. https://doi.org/10.1016/S0009-2614(00)00757-0
Lin BC, Cheng CP, You Z-Q, Hsu C-P (2005) Charge transport properties of tris(8-hydroxyquinolinato)aluminum(III): why it is an electron transporter. J Am Chem Soc 127:66–67. https://doi.org/10.1021/ja045087t
Irfan A, Cui R, Zhang J (2010) Toward rational designing of n-type materials: theoretical investigations of mer-Alq3 derivatives. J Mol Struct (Thoechem) 956:61–65. https://doi.org/10.1016/j.theochem.2010.06.028
Shi Y, Chang Y, Lu K, Chen Z, Zhang J, Yan Y, Qiu D, Liu Y, Adil MA, Ma W, Hao X, Zhu L, Wei Z (2022) Small reorganization energy acceptors enable low energy losses in non-fullerene organic solar cells. Nat Commun 13:3256. https://doi.org/10.1038/s41467-022-30927-y
Wu C-C, Li EY, Chou P-T (2022) Reducing the internal reorganization energy via symmetry controlled π-electron delocalization. Chem Sci 13:7181–7189. https://doi.org/10.1039/D2SC01851A
Acknowledgements
We gratefully acknowledge Professor Dr. P. M. ANBARASAN (Periyar University Salem) for his invaluable guidance and computational support.
Author information
Authors and Affiliations
Contributions
K. DEEPAKVIJAY: The first author played a pivotal role in this research project, providing the foundational idea, conducting quantum chemical calculations, and writing the manuscript.
A. PRAKASAM: The second and corresponding author provided guidance, supervision, and manuscript review, ensuring the project’s quality.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deepakvijay, K., Prakasam, A. Exploring the effects of mono-bromination on hole-electron transport and distribution in dibenzofuran and dibenzothiophene isomers: a first-principles study. J Mol Model 30, 171 (2024). https://doi.org/10.1007/s00894-024-05966-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-024-05966-5