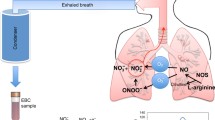
Abstract
Context
Coronavirus (COVID-19) is a novel respiratory viral infection, causing a relatively large number of deaths especially in people who underly lung diseases such as chronic obstructive pulmonary and asthma, and humans are still suffering from the limited testing capacity. In this article, a solution is proposed for the detection of COVID-19 viral infections through the analysis of exhaled breath gasses, i.e., nitric oxide, a prominent biomarker released by respiratory epithelial, as a non-invasive and time-saving approach. Here, we designed a novel and low-cost N and P co-doped C60 fullerene-based breathalyzer for the detection of NO gas exhaled from the respiratory epithelial cells. This breathalyzer shows a quick response to the detection of NO gas by directly converting NO to NO2 without passing any energy barrier (0 kcal/mol activation energy). The recovery time of breathalyzer is very short (0.98 × 103 s), whereas it is highly selective for NO sensing in the mixture of CO2 and H2O gasses. The study provides an idea for the synthesis of low-cost (compared to previously reported Au atom decorated nanostructure and metal-based breathalyzer), efficient, and highly selective N and P co-doped C60 fullerene-based breathalyzer for COVID-19 detection.
Methods
The geometries of N and P-doped systems and gas molecules are simulated using spin-polarized density functional theory calculations.
Graphical Abstract







Similar content being viewed by others
Data availability
All relevant data generated or analyzed during the work are included in this paper and any additional data can be made available upon reasonable request.
References
Zhonghua Liu Xing Bing Xue Za Zhi (2020) The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. 41. https://doi.org/10.3760/cma.j.issn.0254-6450.2020.02.003
Ge C, Li M, Li M, Peyghan AA (2020) Au-decorated BN nanotube as a breathalyzer for potential medical applications. J Mol Liq 312:113454. https://doi.org/10.1016/j.molliq.2020.113454
Li F (2013) Receptor recognition and cross-species infections of SARS coronavirus. Antiviral Res 100(1):246–254. https://doi.org/10.1016/j.antiviral.2013.08.014
Li W, Wong SK, Li F, Kuhn JH, Huang IC, Choe H, Farzan M (2006) Animal origins of the severe acute respiratory syndrome coronavirus: insight from ACE2-S-protein interactions. J Virol 80(9):4211–4219. https://doi.org/10.1128/jvi.80.9.4211-4219.2006
Nagy PD, Simon AE (1997) New insights into the mechanisms of RNA recombination. Virology 235(1):1–9. https://doi.org/10.1006/viro.1997.8681
Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C et al (2020) Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8(4):420–422. https://doi.org/10.1016/S2213-2600(20)30076-X
Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW (2003) Treatment of SARS with human interferons. Lancet 362(9380):293–294. https://doi.org/10.1016/S0140-6736(03)13973-6
Chan JF, Chan KH, Kao RY, To KK, Zheng BJ, Li CP et al (2013) Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J Infect 67(6):606–616. https://doi.org/10.1016/j.jinf.2013.09.029
Cheng KW, Cheng SC, Chen WY, Lin MH, Chuang SJ, Cheng IH et al (2015) Thiopurine analogs and mycophenolic acid synergistically inhibit the papain-like protease of Middle East respiratory syndrome coronavirus. Antivir Res 115:9–16. https://doi.org/10.1016/j.antiviral.2014.12.011
Wang Y, Sun Y, Wu A, Xu S, Pan R, Zeng C et al (2015) Coronavirus nsp10/nsp16 methyltransferase can be targeted by nsp10-derived peptide in vitro and in vivo to reduce replication and pathogenesis. J Virol 89(16):8416–8427. https://doi.org/10.1128/jvi.00948-15
Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw FM, Lim WS et al (2015) The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis 211(1):80–90. https://doi.org/10.1093/infdis/jiu396
Gouma PI, Wang L, Simon SR, Stanacevic M (2017) Novel isoprene sensor for a flu virus breath monitor. Sensors 17(1):199. https://doi.org/10.3390/s17010199
Mashir A, Paschke KM, Van Duin D, Shrestha NK, Laskowski D, Storer MK et al (2011) Effect of the influenza a (H1N1) live attenuated intranasal vaccine on nitric oxide (FENO) and other volatiles in exhaled breath. J Breath Res 5(3):037107. https://doi.org/10.1088/1752-7155/5/3/037107
Phillips M, Cataneo RN, Chaturvedi A, Danaher PJ, Devadiga A, Legendre DA et al (2010) Effect of influenza vaccination on oxidative stress products in breath. J Breath Res 4(2):026001. https://doi.org/10.1088/1752-7155/4/2/026001
Kamel KMM, Morsali A, Raissi H (2020) Theoretical insights into the intermolecular and mechanisms of covalent interaction of flutamide drug with COOH and COCl functionalized carbon nanotubes: a DFT approach. Chem Rev Lett 3:23–37
Dos Santos RB, Rivelino R, de Brito Mota F, Gueorguiev GK, Kakanakova-Georgieva A (2015) Dopant species with Al–Si and N–Si bonding in the MOCVD of AlN implementing trimethylaluminum, ammonia and silane. J Phys D Appl Phys 48(29):295104. https://doi.org/10.1088/0022-3727/48/29/295104
Rostamoghli KJR, Vakili M, Banaei A, Pourbashir E (2018) Applying the B12N12 nanoparticle as the CO, CO2, H2O and NH3 sensor. Chem Rev Lett 1:31–36
Beheshtian J, Peyghan AA, Tabar MB, Bagheri Z (2013) DFT study on the functionalization of a BN nanotube with sulfamide. Appl Surf Sci 266:182–187. https://doi.org/10.1016/j.apsusc.2012.11.128
GhafurRauf MSH, Majedi S, E. Abdul kareemMahmood, (2019) Adsorption behavior of the Al- and Ga-doped B12N12 nanocages on COn (n = 1, 2) and HnX (n = 2, 3 and X = O, N): a comparative study. Chem Rev Lett 2:140–150
Freitas RRQ, Gueorguiev GK, de Brito Mota F, De Castilho CMC, Stafström S, Kakanakova-Georgieva A (2013) Reactivity of adducts relevant to the deposition of hexagonal BN from first-principles calculations. Chem Phys Lett 583:119–124. https://doi.org/10.1016/j.cplett.2013.07.077
Babanezhad ABE (2018) The possibility of selective sensing of the straight-chain alcohols (including methanol to n-pentanol) by using the C20 fullerene and C18NB nano cage. Chem Rev Lett 1:82–88
Li F, Asadi H (2020) DFT study of the effect of platinum on the H2 gas sensing performance of ZnO nanotube: explaining the experimental observations. J Mol Liq 309:113139. https://doi.org/10.1016/j.molliq.2020.113139
Siadati SRSA (2018) Switching behavior of an actuator containing germanium, silicon-decorated and normal C20 fullerene. Chem Rev Lett 1:77–81
Gouma P, Prasad A, Stanacevic S (2011) A selective nanosensor device for exhaled breath analysis. J Breath Res 5(3):037110. https://doi.org/10.1088/1752-7155/5/3/037110
Gouma PI, Prasad AK, Iyer KK (2006) Selective nanoprobes for ‘signalling gases’. Nanotechnology 17(4):S48. https://doi.org/10.1088/0957-4484/17/4/008
Huang J, Wan Q (2009) Gas sensors based on semiconducting metal oxide one-dimensional nanostructures. Sensors 9(12):9903–9924. https://doi.org/10.3390/s91209903
He YLY, Chen H-Y, Hou J (2010) Indene−C60 bisadduct: a new acceptor for high-performance polymer solar cells. J Am Chem Soc 132:1377–1382
Yuan YY, Han S, Grozea D, Lu AZ (2006) Fullerene-organic nanocomposite: a flexible material platform for organic light-emitting diodes. Appl Phys Lett 88(9). https://doi.org/10.1063/1.2180876
Sutradhar S, Patnaik A (2017) A new fullerene-C60–Nanogold composite for non-enzymatic glucose sensing. Sensors Actuators B Chem 241:681–689. https://doi.org/10.1016/j.snb.2016.10.111
Esrafili MD, Mohammadirad N (2017) A first-principles study on the adsorption behaviour of methanol and ethanol over C59B heterofullerene. Mol Phys 115(14):1633–1641. https://doi.org/10.1080/00268976.2017.1311423
Moradi M, Nouraliei M, Moradi R (2017) Theoretical study on the phenylpropanolamine drug interaction with the pristine, Si and Al doped [60] fullerenes. Physica E: Low-Dimens Syst Nanostructures 87:186–191. https://doi.org/10.1016/j.physe.2016.11.027
Ganji MD, Yazdani H (2010) Interaction between B-doped C60 fullerene and glycine amino acid from first-principles simulation. Chin Phys Lett 27(4):043102. https://doi.org/10.1088/0256-307X/27/4/043102
Khan AA, Ahmad I, Ahmad R (2020) Influence of electric field on CO2 removal by P-doped C60-fullerene: a DFT study. Chem Phys Lett 742:137155. https://doi.org/10.1016/j.cplett.2020.137155
Vinit, Ramachandran CN (2017) Structure, stability, and properties of boron encapsulated complexes of C60, C59B, and C59N. J Phys Chem A 121(8):1708–1714. https://doi.org/10.1021/acs.jpca.6b10649
Liang Z, Liu C, Chen M, Qi X, Kumar P, Peera SG et al (2019) Oxygen reduction reaction mechanism on P, N co-doped graphene: a density functional theory study. New J Chem 43(48):19308–19317. https://doi.org/10.1039/c9nj04808a
Han C, Chen Z (2020) The mechanism study of oxygen reduction reaction (ORR) on non-equivalent P, N co-doped graphene. Appl Surf Sci 511:145382. https://doi.org/10.1016/j.apsusc.2020.145382
Delley B (1990) An all-electron numerical method for solving the local density functional for polyatomic molecules. J Chem Phys 92:508–517. https://doi.org/10.1063/1.458452
Delley B (2000) From molecules to solids with the DMol3 approach. J Chem Phys 113:7756–7764. https://doi.org/10.1063/1.1316015
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868. https://doi.org/10.1103/PhysRevLett.77.3865
Grimme S (2004) Accurate description of van der Waals complexes by density functional theory including empirical corrections. J Comput Chem 25:1463–1473. https://doi.org/10.1002/jcc.20078
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27:1787–1799. https://doi.org/10.1002/jcc.20495
Liu P, Rodriguez JA (2005) Catalysts for hydrogen evolution from the [NiFe] hydrogenase to the Ni 2P(001) surface: the importance of ensemble effect. J Am Chem Soc 127:14871–14878. https://doi.org/10.1021/ja0540019
Yong Y, Cui H, Zhou Q, Su X, Kuang Y, Li X (2019) C2N monolayer as NH3 and NO sensors: a DFT study. Appl Surf Sci 487:488–495. https://doi.org/10.1016/j.apsusc.2019.05.040
Khan AA, Ahmad R, Ahmad I (2020) Density functional theory study of emerging pollutants removal from water by covalent triazine based framework. J Mol Liq 309:113008. https://doi.org/10.1016/j.molliq.2020.113008
Ma S, Su L, Jin L, Su J, Jin Y (2019) A first-principles insight into Pd-doped MoSe2 monolayer: a toxic gas scavenger. Phys Lett A 383(30):125868. https://doi.org/10.1016/j.physleta.2019.125868
Acknowledgements
The authors would like to thank “Center for Computational Materials Science of University of Malakand” for its technical support of this work.
Author information
Authors and Affiliations
Contributions
Adnan Ali Khan: conceptualization, methodology, software, writing—original draft, writing—review and editing. Fazal Mehmood: formal analysis, validation, writing—original draft. Rashid Ahmad: supervision, conceptualization, software, writing—review and editing. Iftikhar Ahmad: conceptualization, software, project administration.
Corresponding authors
Ethics declarations
Ethics approval
This research did not involve any human participants or animals to generate and analyze the data used in this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khan, A.A., Ahmad, R., Mehmood, F. et al. Efficient detection of nitric oxide a biomarker associated with COVID19 via N, P co-doped C60 fullerene: a computational study. J Mol Model 30, 166 (2024). https://doi.org/10.1007/s00894-024-05954-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-024-05954-9