Abstract
Context
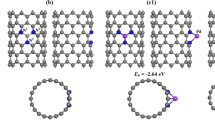
CO2 and CO gas sensors are very important to recognize the insulation situation of electrical tools. ToCO explore the application of noble metal doped of aluminum nitride nanotubes for gas sensors, DFT computations according to the first principal theory were applied to study sensitivity, adsorption attributes, and electronic manner. In this investigation, platinum-doped aluminum nitride nanotubes were offered for the first time to analyze the adsorption towards CO2 and CO gases. Firm construction of platinum-doped aluminum nitride nanotubes (Pt-AlNNT) was investigated in four feasible places, and the binding energy of firm construction is 1.314 eV. Respectively, the adsorption energy between the CO2 and Pt-AlNNT systems was − 2.107 eV, while for instance of CO, the adsorption energy was − 3.258 eV. The mentioned analysis and computations are considerable for studying Pt-AlNNT as a new CO2 and CO gas sensor for electrical tools insulation. The current study revealed that the Pt-AlNNT possesses high selectivity and sensitivity towards CO2 and CO.
Methods
In this research, Pt-doped AlNNT (Pt-AlNNT) has been studied as sensing materials of CO and CO2 for the first time. The adsorption process of Pt-AlNNT has been computed and analyzed through the DFT approach. DFT computations by using B3LYP functional and 6–31 + G* basis sets have been applied in the GAMESS code for sensing attributes, which contribute to potential applications.





Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Haija MA, Ayesh AI, Ahmed S, Katsiotis MS (2016) Selective hydrogen gas sensor using CuFe2O4 nanoparticle based thin film. Appl Surf Sci 369:443–447
Zhou Q, Xu L, Umar A, Chen W, Kumar R (2018) Pt nanoparticles decorated SnO2 nanoneedles for efficient CO gas sensing applications. Sens Actuators, B Chem 256:656–664
Abbasi A, Sardroodi JJ (2017) An innovative gas sensor system designed from a sensitive nanostructured ZnO for the selective detection of SO x molecules: a density functional theory study. New J Chem 41:12569–12580
Jiang Z, Han X, Zhao C, Wang S, Tang X (2022) Recent advance in biological responsive nanomaterials for biosensing and molecular imaging application. Int J Mol Sci 23:1923
Wang Z, Ma R, Chen B, Yu X, Wang X, Zuo X, Liang B, Yang J (2024) A transcription factor-based bacterial biosensor system and its application for on-site detection of explosives. Biosens Bioelectron 244:115805
Takeda H, Ueda T, Kamada K, Matsuo K, Hyodo T, Shimizu Y (2015) CO-sensing properties of a NASICON-based gas sensor attached with Pt mixed with Bi2O3 as a sensing electrode. Electrochim Acta 155:8–15
Zhao W, Fam DWH, Yin Z, Sun T, Tan HT, Liu W, Tok AIY, Boey YCF, Zhang H, Hng HH (2012) A carbon monoxide gas sensor using oxygen plasma modified carbon nanotubes. Nanotechnology 23:425502
Abbasi A (2019) Modulation of the electronic properties of pristine and AlP-codoped stanene monolayers by the adsorption of CH2O and CH4 molecules: a DFT study. Mater Res Exp 6:076410
Osouleddini N, Rastegar SF (2019) DFT study of the CO2 and CH4 assisted adsorption on the surface of graphene. J Electron Spectrosc Relat Phenom 232:105–110
Montzka SA, Dlugokencky EJ, Butler JH (2011) Non-CO2 greenhouse gases and climate change. Nature 476:43–50
Lashof DA, Ahuja DR (1990) Relative contributions of greenhouse gas emissions to global warming. Nature 344:529–531
Li M, Lv S, Yang R, Chu X, Wang X, Wang Z, Peng L, Yang J (2023) Development of lycopene-based whole-cell biosensors for the visual detection of trace explosives and heavy metals. Anal Chim Acta 1283:341934
Wang N, Li X, Lian X, Zhuang Q, Wang J, Li J, Qian H, Miao K, Wang Y, Luo X (2024) Acetate ions facilitated immobilization of highly dispersed transition metal oxide nanoclusters in mesoporous silica. Inorg Chem 63(9):4393–4403
Esrafili MD (2019) Electric field assisted activation of CO2 over P-doped graphene: a DFT study. J Mol Graph Model 90:192–198
Abbasi A, Sardroodi JJ (2019) Adsorption of O3, SO2 and SO3 gas molecules on MoS2 monolayers: a computational investigation. Appl Surf Sci 469:781–791
Cui H, Zhang X, Zhang J, Zhang Y (2019) Nanomaterials-based gas sensors of SF6 decomposed species for evaluating the operation status of high-voltage insulation devices. High Voltage 4:242–258
Ma S, Li D, Rao X, Xia X, Su Y, Lu Y (2020) Pd-doped h-BN monolayer: a promising gas scavenger for SF6 insulation devices. Adsorption 26:619–626
Abbasi A, Sardroodi JJ, Ebrahimzadeh AR, Yaghoobi M (2018) Theoretical study of the structural and electronic properties of novel stanene-based buckled nanotubes and their adsorption behaviors. Appl Surf Sci 435:733–742
Abbasi A, JahanbinSardroodi J (2018) Interaction of sulfur trioxide molecules with armchair and zigzag stanene-based nanotubes: electronic properties exploration by DFT calculations. Adsorption 24:443–458
Zhang X, Yu L, Wu X, Hu W (2015) Experimental sensing and density functional theory study of H2S and SOF2 adsorption on Au-modified graphene. Adv Sci 2:1500101
Zhang X, Chen D, Cui H, Dong X, Xiao S, Tang J (2017) Understanding of SF6 decompositions adsorbed on cobalt-doped SWCNT: a DFT study. Appl Surf Sci 420:371–382
Zhang X, Dong X, Gui Y (2016) Theoretical and experimental study on competitive adsorption of SF6 decomposed components on Au-modified anatase (101) surface. Appl Surf Sci 387:437–445
Kim P, Lieber CM (1999) Nanotube nanotweezers. Science 286:2148–2150
Tans SJ, Verschueren AR, Dekker C (1998) Room-temperature transistor based on a single carbon nanotube. Nature 393:49–52
Li Y-H, Hung T-H, Chen C-W (2009) A first-principles study of nitrogen-and boron-assisted platinum adsorption on carbon nanotubes. Carbon 47:850–855
Yue X, Ma NL, Sonne C, Guan R, Lam SS, Van Le Q, Chen X, Yang Y, Gu H, Rinklebe J (2021) Mitigation of indoor air pollution: a review of recent advances in adsorption materials and catalytic oxidation. J hazard Mater 405:124138
Liu Z, Lin X, Lee JY, Zhang W, Han M, Gan LM (2002) Preparation and characterization of platinum-based electrocatalysts on multiwalled carbon nanotubes for proton exchange membrane fuel cells. Langmuir 18:4054–4060
Tang H, Chen J, Huang Z, Wang D, Ren Z, Nie L, Kuang Y, Yao S (2004) High dispersion and electrocatalytic properties of platinum on well-aligned carbon nanotube arrays. Carbon 42:191–197
Wang Q, Setlur A, Lauerhaas J, Dai J, Seelig E, Chang R (1998) A nanotube-based field-emission flat panel display. Appl Phys Lett 72:2912–2913
Peng S, Cho K (2003) Ab initio study of doped carbon nanotube sensors. Nano Lett 3:513–517
Abbasi A, Khataee A (2019) Band gap tunability and structural stability of metal/nonmetal codoped group-IV tin nanotubes: effect of spin-orbit coupling. Physica E 114:113644
Abbasi AJ, Sardroodi J (2018) Structural and electronic properties of group-IV tin nanotubes and their effects on the adsorption of SO2 molecules: insights from DFT computations. J Appl Phys 124(16):165302
Ahmed R, Hashemifar SJ, Akbarzadeh H (2008) First-principles study of the structural and electronic properties of III-phosphides. Physica B 403:1876–1881
Hou S, Shen Z, Zhang J, Zhao X, Xue Z (2004) Ab initio calculations on the open end of single-walled BN nanotubes. Chem Phys Lett 393:179–183
Zhang D, Zhang R (2003) Theoretical prediction on aluminum nitride nanotubes. Chem Phys Lett 371:426–432
Abbasi A (2019) Adsorption of phenol, hydrazine and thiophene on stanene monolayers: a computational investigation. Synth Met 247:26–36
Wang J, Wang P, Chen W, Wan F, Lu Y, Tang Z, Dong A, Lei Z, Zhang Z (2023) Highly sensitive multi-pass cavity enhanced Raman spectroscopy with novel polarization filtering for quantitative measurement of SF6 decomposed components in gas-insulated power equipment. Sens Actuators, B Chem 380:133350
Erkoç Ş (2004) Semi-empirical SCF-MO calculations for the structural and electronic properties of single-wall InP nanotubes. J Mol Struct (Thoechem) 676:109–113
Jain S, Willander M, Narayan J, Overstraeten RV (2000) III–nitrides: growth, characterization, and properties. J Appl Phys 87:965–1006
Bagheri Z, Mirzaei M, Hadipour N, Abolhassani M (2008) Density functional theory study of boron nitride nanotubes: calculations of the N-14 and B-11 nuclear quadrupole resonance parameters. J Comput Theor Nanosci 5:614–618
Qian Z, Hou S, Zhang J, Li R, Shen Z, Zhao X, Xue Z (2005) Stability and electronic structure of single-walled InN nanotubes. Physica E 30:81–85
Tondare V, Balasubramanian C, Shende S, Joag D, Godbole V, Bhoraskar S, Bhadbhade M (2002) Field emission from open ended aluminum nitride nanotubes. Appl Phys Lett 80:4813–4815
Liu W-G, Chen G-H, Huang X-C, Wu D, Yu Y-P (2012) DFT studies on the interaction of an open-ended single-walled aluminum nitride nanotube (AlNNT) with gas molecules. The Journal of Physical Chemistry C 116:4957–4964
Rezouali K, Belkhir MA, Houari A, Bai J (2009) Ab initio study of confinement and surface effects in hexagonal AlN nanotubes. Comput Mater Sci 45:305–309
Streicher E, Chartier T, Boch P, Denanot M-F, Rabier J (1990) Densification and thermal conductivity of low-sintering-temperature AlN materials. J Eur Ceram Soc 6:23–29
Wu C, Kahn A (2000) Negative electron affinity and electron emission at cesiated GaN and AlN surfaces. Appl Surf Sci 162:250–255
Blase X, Rubio A, Louie S, Cohen M (1994) Stability and band gap constancy of boron nitride nanotubes. EPL (Europhysics Letters) 28:335
Abbasi A, Sardroodi JJ (2018) Density functional theory investigation of the interactions between the buckled stanene nanosheet and XO2 gases (X = N, S, C). Comput Theor Chem 1125:15–28
Abbasi A, Sardroodi JJ (2019) The adsorption of sulfur trioxide and ozone molecules on stanene nanosheets investigated by DFT: applications to gas sensor devices. Physica E 108:382–390
Abbasi A (2019) Theoretical investigation of the interaction between noble metals (Ag, Au, Pd, Pt) and stanene nanosheets: a DFT study. J Inorg Organomet Polym Mater 29:1895–1915
Choi CH, Kim M, Kwon HC, Cho SJ, Yun S, Kim H-T, Mayrhofer KJ, Kim H, Choi M (2016) Tuning selectivity of electrochemical reactions by atomically dispersed platinum catalyst. Nat Commun 7:1–9
da Rocha CG, Clayborne PA, Koskinen P, Häkkinen H (2014) Optical and electronic properties of graphene nanoribbons upon adsorption of ligand-protected aluminum clusters. Phys Chem Chem Phys 16:3558–3565
Babanezhad E, Beheshti A (2018) The possibility of selective sensing of the straight-chain alcohols (including methanol to n-pentanol) using the C20 fullerene and C18NB nano cage. Chemical Review and Letters 1:82–88
Ghafur Rauf H, Majedi S, Abdulkareem Mahmood E, Sofi M (2019) Adsorption behavior of the Al- and Ga-doped B12N12 nanocages on COn (n=1, 2) and HnX (n=2, 3 and X=O, N): a comparative study. ChemRev Lett 2:140–150
Chen D, Zhang X, Xiong H, Li Y, Tang J, Xiao S, Zhang D (2019) A first-principles study of the SF6 decomposed products adsorbed over defective WS2 monolayer as promising gas sensing device. IEEE Trans Device Mater Reliab 19:473–483
Zhang D, Wu J, Li P, Cao Y (2017) Room-temperature SO2 gas-sensing properties based on a metal-doped MoS2 nanoflower: an experimental and density functional theory investigation. J Mater Chem A 5:20666–20677
Zhang D, Yang Z, Li P, Pang M, Xue Q (2019) Flexible self-powered high-performance ammonia sensor based on Au-decorated MoSe2 nanoflowers driven by single layer MoS2-flake piezoelectric nanogenerator. Nano Energy 65:103974
Zhang D, Wu J, Li P, Cao Y, Yang Z (2019) Hierarchical nanoheterostructure of tungsten disulfide nanoflowers doped with zinc oxide hollow spheres: benzene gas sensing properties and first-principles study. ACS Appl Mater Interfaces 11:31245–31256
Becke AD (1992) Density‐functional thermochemistry. I. The effect of the exchange‐only gradient correction. J Chem Phys 96:2155–2160
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785
Abbasi A, Sardroodi JJ (2018) A highly sensitive chemical gas detecting device based on N-doped ZnO as a modified nanostructure media: a DFT+NBO analysis. Surf Sci 668:150–163
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors, Molecular Physics 19:553–566
Leenaraj DR, Joe IH (2015) Natural Bond Orbital Analysis and DFT Calculation of Non-opiod Analgesic Drug Lidocaine. Mater Today: Proc 2:969–972
Ehlers AW, Baerends EJ, Lammertsma K (2002) Nucleophilic or electrophilic phosphinidene complexes ML n PH; what makes the difference? J Am Chem Soc 124:2831–2838
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S (1993) General atomic and molecular electronic structure system. J Comput Chem 14:1347–1363
Shahabi M, Raissi H (2016) Investigation of the molecular structure, electronic properties, AIM, NBO, NMR and NQR parameters for the interaction of Sc, Ga and Mg-doped (6,0) aluminum nitride nanotubes with COCl2 gas by DFT study. J Incl Phenom Macrocycl Chem 84:99–114
Salih E, Ayesh AI (2020) CO, CO2, and SO2 detection based on functionalized graphene nanoribbons: first principles study. Physica E 123:114220
Zheng Z, Wang H (2019) Different elements doped graphene sensor for CO2 greenhouse gases detection: the DFT study. Chem Phys Lett 721:33–37
Acknowledgements
This work was supported by Princess Nourah bint Abdulrahman University researchers supporting project number (PNURSP2024R205), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Author Contributions Statement: M.J. Saadh, A.T Ahmed, A. Mahal: Conceptualization, Methodology, Software, Writing, Conceptualization, Methodology, Management and responsibility for the research activity planning and execution; S. Chandra, M.A. Almajed, H. F. Alotaibi, A.M. Hamoody: Methodology, Software, Writing—review & editing; M.N. Shakir. Ahmed, R. Zainul: Writing—original draft, Methodology, Software, review & editing.
Corresponding author
Ethics declarations
Ethical approval
Not required.
Consent to participate
Confirm.
Consent for publication
Confirm.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saadh, M.J., Ahmed, A.T., Mahal, A. et al. Assessing the gas sensing capability of undoped and doped aluminum nitride nanotubes. J Mol Model 30, 153 (2024). https://doi.org/10.1007/s00894-024-05953-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-024-05953-w