Abstract
Context
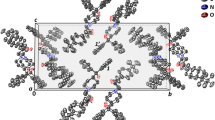
The advancement in the development of second-generation drugs in the field of antihistamines represents a significant milestone in the management of allergic diseases, targeting the effects of histamine. The efficacy of bilastine in treating allergic disorders has sparked interest in investigating its polymorphism, a crucial property that impacts quality, safety, and effectiveness as per regulatory guidelines. This study examines the polymorphism of bilastine, focusing on two crystalline forms labeled as Form I and Form II. Utilizing advanced analytical techniques, the research explores the structural characteristics and molecular interactions within these forms. Geometric parameters, such as bond lengths, bond angles, and torsion angles, are examined to comprehend molecular conformations and crystal packing arrangements. Hydrogen bonding, covalent bonds, and van der Waals forces contribute to the unique supramolecular arrangements in these forms. This study provides a significant contribution to understanding bilastine’s polymorphism, offering critical insights to researchers and regulatory bodies to ensure the quality, efficacy, and safety of antihistamine products.
Methods
The molecular conformation of two bilastine forms was obtained through DFT with the exchange–correlation functional M06-2X and the 6–311 + + G(d,p) basis set, and the results were compared with the experimental X-ray. The atomic coordinates were obtained directly from the crystalline structures, and charge transfer was also investigated using frontier molecular orbitals (HOMO and LUMO), and MEP map in order to evaluate the energies associated with charge transfers and regions of high electron affinity. The geometric and topological parameters and intermolecular interactions in the crystals were analyzed using Hirshfeld Surface.










Similar content being viewed by others
Data availability
No.
Code availability
N/A.
References
Li L, Liu R, Peng C, Chen X, Li J (2022) Pharmacogenomics for the efficacy and side effects of antihistamines. Exp Dermatol 31:993–1004
Simons FER (2004) Advances in H 1-Antihistamines. N Engl J Med 351:2203–2217
Church MK (2011) Safety and efficacy of bilastine: a new H1-antihistamine for the treatment of allergic rhinoconjunctivitis and urticaria. Expert Opin Drug Saf 10:779–793
Church MK, Tiongco-Recto M, Ridolo E, Novák Z (2020) Bilastine: a lifetime companion for the treatment of allergies. Curr Med Res Opin 36:445–454
Prathyusha P, Sundararajan R, Bhanu P, Mukthinuthalapati MA. (2020) A new stability indicating RP-HPLC method for determination of bilastine in bulk and pharmaceutical formulation. Res J Pharm Technol;13. Epub ahead of print. https://doi.org/10.5958/0974-360X.2020.00507.7.
Jáuregui I, Ferrer M, Bartra J, del Cuvillo A, Dávila I et al (2013) Bilastine for the treatment of urticaria. Expert Opin Pharmacother 14:1537–1544
Eb FORD (1945) Polymorphism. Biol Rev 20:73–88
Purohit R, Venugopalan P. (2009) Polymorphism: an overview. Resonance;14. Epub ahead of print. https://doi.org/10.1007/s12045-009-0084-7.
Singh Randhawa A, Mohd Noor N, Md Daud MK, Abdullah B. (2022) Efficacy and safety of bilastine in the treatment of allergic rhinitis: a systematic review and meta-analysis. Frontiers in Pharmacology;12. Epub ahead of print. https://doi.org/10.3389/fphar.2021.731201.
Nechipadappu SK, Swain D. (2023) Combined synthetic and solubility aspects of orotate salt of bilastine. J Mol Struct;1271. Epub ahead of printx. https://doi.org/10.1016/j.molstruc.2022.134148.
Groom CR, Bruno IJ, Lightfoot MP, Ward SC. (2016) The Cambridge structural database. Acta Crystallogr B Struct Sci Cryst Eng Mater;72. Epub ahead of print. https://doi.org/10.1107/S2052520616003954.
Puschmann H, Bourhis LJ, Dolomanov O V, Gildea RJ, Howard JAK. (2013) OLEX2–a complete package for molecular crystallography. Acta Crystallogr A;69. Epub ahead of print. https://doi.org/10.1107/s0108767313094026.
Dolomanov O V, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H. (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Crystallogr;42. Epub ahead of print. https://doi.org/10.1107/S0021889808042726.
Farrugia LJ (2012) WinGX and ORTEP for Windows : an update. J Appl Crystallogr 45:849–854
Macrae CF, Sovago I, Cottrell SJ, Galek PTA, McCabe P et al (2020) Mercury 4.0: from visualization to analysis, design and prediction. J Appl Crystallogr 53:226–235
Hirshfeld FL. (1977) Bonded-atom fragments for describing molecular charge densities. Theor Chim Acta;44. Epub ahead of print. https://doi.org/10.1007/BF00549096.
McKinnon JJ, Spackman MA, Mitchell AS. (2004) Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crystallographica Section B: Structural Science;60. Epub ahead of print. https://doi.org/10.1107/S0108768104020300.
Spackman MA, McKinnon JJ. (2002) Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm;4. Epub ahead of print. https://doi.org/10.1039/b203191b.
Spackman MA, Jayatilaka D. (2009) Hirshfeld surface analysis. CrystEngComm;11. Epub ahead of print. https://doi.org/10.1039/b818330a.
Jelsch C, Ejsmont K, Huder L. (2014) The enrichment ratio of atomic contacts in crystals, an indicator derived from the Hirshfeld surface analysis. IUCrJ;1. Epub ahead of print. https://doi.org/10.1107/S2052252514003327.
McKinnon JJ, Fabbiani FPA, Spackman MA. (2007) Comparison of polymorphic molecular crystal structures through Hirshfeld surface analysis. Cryst Growth Des;7. Epub ahead of print. https://doi.org/10.1021/cg060773k.
McKinnon JJ, Jayatilaka D, Spackman MA. (2007) Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chemical Communications. Epub ahead of print. https://doi.org/10.1039/b704980c.
Spackman PR, Turner MJ, McKinnon JJ, Wolff SK, Grimwood DJ et al (2021) CrystalExplorer: a program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J Appl Crystallogr 54:1006–1011
Frisch MJ (2009) GAUSSIAN09. Gaussian Inc, Wallingford, CT, USA
Zhao Y, Truhlar DG. (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc;120. Epub ahead of print. https://doi.org/10.1007/s00214-007-0310-x.
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241
Gber TE, Louis H, Owen AE, Etinwa BE, Benjamin I et al (2022) Heteroatoms (Si, B, N, and P) doped 2D monolayer MoS 2 for NH 3 gas detection. RSC Adv 12:25992–26010
Santin LG, Toledo EM, Carvalho-Silva VH, Camargo AJ, Gargano R et al (2016) Methanol solvation effect on the proton rearrangement of curcumin’s enol forms: an ab initio molecular dynamics and electronic structure viewpoint. J Physical Chem C 120:19923–19931
Ríos‐Gutiérrez M, Saz Sousa A, Domingo LR. (2023) Electrophilicity and nucleophilicity scales at different DFT computational levels. J Phys Org Chem;36. Epub ahead of print. https://doi.org/10.1002/poc.4503.
Aguiar ASN, Queiroz JE, Firmino PP, Vaz WF, Camargo AJ et al (2020) Synthesis, characterization, and computational study of a new heteroaryl chalcone. J Mol Model 26:243
Tosoni S, Tuma C, Sauer J, Civalleri B, Ugliengo P. (2007) A comparison between plane wave and Gaussian-type orbital basis sets for hydrogen bonded systems: formic acid as a test case. J Chem Phys;127. Epub ahead of print 21 October. https://doi.org/10.1063/1.2790019.
Bakheit AH, Abuelizz HA, Al-Salahi R (2023) Hirshfeld surface analysis and Density functional theory calculations of 2-benzyloxy-1,2,4-triazolo[1,5-a] quinazolin-5(4H)-one: a comprehensive study on crystal structure, intermolecular interactions, and electronic properties. Crystals (Basel) 13:1410
Bakheit AH, Alkahtani HM (2023) Integrated structural, functional, and ADMET analysis of 2-methoxy-4,6-diphenylnicotinonitrile: the convergence of X-ray diffraction, molecular docking, dynamic simulations, and advanced computational insights. Molecules 28:6859
Zhang G, Musgrave CB. (2007) Comparison of DFT methods for molecular orbital eigenvalue calculations. J Physical Chem A;111. Epub ahead of print. https://doi.org/10.1021/jp061633o.
Weiner PK, Langridge R, Blaney JM, Schaefer R, Kollman PA. (1982) Electrostatic potential molecular surfaces. Proc Natl Acad Sci U S A;79. Epub ahead of print. https://doi.org/10.1073/pnas.79.12.3754.
Náray-Szabó G, Ferenczy GG. (1995) Molecular electrostatics. Chem Rev;95. Epub ahead of print. https://doi.org/10.1021/cr00036a002.
Per S, Peter P (1990) Use of the electrostatic potential at the molecular surface to interpret and predict nucleophilic processes. J Phys Chem 94:3959–3961
Bernstein J. (2020) Polymorphism in molecular crystals 2e. Oxford University Press. Oxford.
Herbstein FH (2004) Diversity amidst similarity: a multidisciplinary approach to phase relationships, solvates, and polymorphs. Cryst Growth Des 4:1419–1429
Bergeron H, Lebedev D, Hersam MC (2021) Polymorphism in post-dichalcogenide two-dimensional materials. Chem Rev 121:2713–2775
Aakeröy CB, Seddon KR (1993) The hydrogen bond and crystal engineering. Chem Soc Rev 22:397–407
Chikalov I, Yao P, Moshkov M, Latombe J-C (2011) Learning probabilistic models of hydrogen bond stability from molecular dynamics simulation trajectories. BMC Bioinformatics 12:S34
Kawahara S, Kojima C, Taira K, Uchimaru T (2003) A theoretical study of correlation between hydrogen-bond stability and J-coupling through a hydrogen bond. Helv Chim Acta 86:3265–3273
Reichel M, Dosch D, Klapötke T, Karaghiosoff K (2019) Correlation between structure and energetic properties of three nitroaromatic compounds: Bis(2,4-dinitrophenyl) ether, Bis(2,4,6-trinitrophenyl) ether, and Bis(2,4,6-trinitrophenyl) thioether. J Am Chem Soc 141:19911–19916
Puigjaner C, Portell A, Blasco A, Font-Bardia M, Vallcorba O (2021) Entrapped transient chloroform solvates of bilastine. Crystals (Basel) 11:342
Rauk A. (2000) Orbital interaction theory of organic chemistry. Wiley; 2000. Epub ahead of print. https://doi.org/10.1002/0471220418.
Acknowledgements
The authors are grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa de Goiás (FAPEG). H.B. Napolitano is a CNPq fellow (grant 302904/2023-9). Theoretical calculations were carried out in the High-Performance Computing Center of the Universidade Estadual de Goiás.
Funding
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico-(CNPq), and Fundação de Amparo à Pesquisa de Goiás (FAPEG).
Author information
Authors and Affiliations
Contributions
A statement of contributions of the authors:
1. Introduction: Lauriane G. Santin, Lara F. Moreira, Nathan V. C. Oliveira, Vitória L. A. Paiva, Marina R. Ribeiro, Solemar S. Oliveira and Hamilton B. Napolitano.
2. Crystallographic analysis: Lara F. Moreira, Nathan V. C. Oliveira, Vitória L. A. Paiva, Marina R. Ribeiro, and Hamilton B. Napolitano.
3. Computational procedure: Lauriane G. Santin and Solemar S. Oliveira.
4. Results and discussion: Lauriane G. Santin, Lara F. Moreira, Nathan V. C. Oliveira, Vitória L. A. Paiva, Marina R. Ribeiro, Solemar S. Oliveira, and Hamilton B. Napolitano.
5. Conclusion: Lauriane G. Santin, Lara F. Moreira, Nathan V. C. Oliveira, Vitória L. A. Paiva, Marina R. Ribeiro, Solemar S. Oliveira, and Hamilton B. Napolitano.
Corresponding author
Ethics declarations
Ethics approval
N/A
Consent to participate
Yes. All the authors contributed and consented to participate in the manuscript.
Consent for publication
Yes. All the authors consent with the publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santin, L.G., Moreira, L.F., Oliveira, N.V.C. et al. Insights on molecular modeling and supramolecular arrangement of bilastine polymorphs. J Mol Model 30, 157 (2024). https://doi.org/10.1007/s00894-024-05951-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-024-05951-y