Abstract
Context
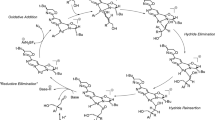
The combined use of transition metal-catalyzed C–H activation with aryne annulation reactions has emerged as an important strategy in organic synthesis. In this study, the mechanisms of the palladium(II)-catalyzed annulation reaction of N-methoxy amides and arynes were computationally investigated by density functional theory. The role of methoxy amide as a directing group was elucidated through the calculation of three different pathways for the C–H activation step, showing that the pathway where amide nitrogen acts as a directing group is preferable. At the reductive elimination transition state, an unstable seven-membered ring is formed preventing the lactam formation. A substituent effect study based on an NBO analysis, Hammet, and using a More O’Ferall-Jenks plot indicates that the C–H activation step proceeds via an electrophilic concerted metalation-deprotonation (eCMD) mechanism. The results show that electron-withdrawing groups increase the activation barrier and contribute to an early Pd–C bond formation and a late C–H bond breaking when compared with electron-donating substituents. Our computational results are in agreement with the experimental data provided in the literature.
Methods
All calculations were performed using Gaussian 16 software. Geometry optimizations, frequency analyses at 393.15 K, and IRC calculations were conducted at the M06L/Def2-SVP level of theory. Corrected electronic energies, NBO charges, and Wiberg bond indexes were computed at the M06L/Def2-TZVP//M06L/Def2-SVP level of theory. Implicit solvent effects were considered in all calculations using the SMD model, with acetonitrile employed as the solvent.











Similar content being viewed by others
Data availability
The inputs and outputs files of the calculations presented in the article are available in the ioChem-BD repository (https://doi.org/10.19061/iochem-bd-6-343). Additional data is provided in the supplementary information.
References
Rogge T, Kaplaneris N, Chatani N, Kim J, Chang S, Punji B, Schafer LL, Musaev DG, Wencel-Delord J, Roberts CA, Sarpong R, Wilson ZE, Brimble MA, Johansson MJ, Ackermann L (2021) C-H activation. Nat Rev Methods Primers 1(1):1–31. https://doi.org/10.1038/s43586-021-00041-2
Sinha SK, Guin S, Maiti S, Biswas JP, Porey S, Maiti D (2022) Toolbox for distal C-H bond functionalizations in organic molecules. Chem Rev 122(6):5682–5841. https://doi.org/10.1021/acs.chemrev.1c00220
Shang W, Sun H, Chen W, Liu J (2023) Diversification of pharmaceutical molecules via late-stage C(sp2)-H functionalization. Green Synth Catal 4(2):104–123. https://doi.org/10.1016/j.gresc.2022.12.007
Dalton T, Faber T, Glorius F (2021) C-H activation: toward sustainability and applications. ACS Cent Sci 7(2):245–261. https://doi.org/10.1021/acscentsci.0c01413
Carvalho RL, Dias GG, Pereira CL, Ghosh P, Maiti D, Silva Júnior ENd (2021) A catalysis guide focusing on c-h activation processes. J Braz Chem Soc 32:917–952. https://doi.org/10.21577/0103-5053.20210025
Bhunia A, Yetra SR, Biju AT (2012) Recent advances in transition-metal-free carbon-carbon and carbon-heteroatom bond-forming reactions using arynes. Chem Soc Rev 41(8):3140–3152. https://doi.org/10.1039/C2CS15310F
Shi J, Li L, Li Y (2021) o-Silylaryl triflates: a journey of Kobayashi aryne precursors. Chem Rev 121(7):3892–4044. https://doi.org/10.1021/acs.chemrev.0c01011
Takikawa H, Nishii A, Sakai T, Suzuki K (2018) Aryne-based strategy in the total synthesis of naturally occurring polycyclic compounds. Chem Soc Rev 47(21):8030–8056. https://doi.org/10.1039/C8CS00350E
Neog K, Borah A, Gogoi P (2016) Palladium(II)-catalyzed C-H bond activation/C-C and C-O bond formation reaction cascade: direct synthesis of coumestans. J Org Chem 81(23):11971–11977. https://doi.org/10.1021/acs.joc.6b01966
Li S, Liu L, Wang R, Yang Y, Li J, Wei J (2020) Palladium-catalyzed oxidative annulation of sulfoximines and arynes by C-H functionalization as an approach to dibenzothiazines. Org Lett 22(19):7470–7474. https://doi.org/10.1021/acs.orglett.0c02615
Yao T, He D (2017) Palladium-catalyzed domino heck/aryne carbopalladation/C-H functionalization: synthesis of heterocycle-fused 9,10-dihydrophenanthrenes. Org Lett 19(4):842–845. https://doi.org/10.1021/acs.orglett.6b03833
Rogness DC, Markina NA, Waldo JP, Larock RC (2012) Synthesis of pyrido[1,2-a]indole malonates and amines through aryne annulation. J Org Chem 77(6):2743–2755. https://doi.org/10.1021/jo2025543
Feng M, Tang B, Xu HX, Jiang X (2016) Collective synthesis of phenanthridinone through C-H activation involving a Pd-catalyzed aryne multicomponent reaction. Org Lett 18(17):4352–4355. https://doi.org/10.1021/acs.orglett.6b02109
Zhao J, Li H, Li P, Wang L (2019) Annulation of benzamides with arynes using palladium with photoredox dual catalysis. J Org Chem 84(14):9007–9016. https://doi.org/10.1021/acs.joc.9b00893
Peng X, Wang W, Jiang C, Sun D, Xu Z, Tung CH (2014) Strain-promoted oxidative annulation of arynes and cyclooctynes with benzamides: palladium-catalyzed C-H/N-H activation for the synthesis of N-heterocycles. Org Lett 16(20):5354–5357. https://doi.org/10.1021/ol5025426
Feng S, Li S, Li J, Wei J (2019) Palladium-catalyzed annulation of N-alkoxy benzsulfonamides with arynes by C-H functionalization: access to dibenzosultams. Org Chem Front 6(4):517–522. https://doi.org/10.1039/C8QO01311J
Tang CY, Wu XY, Sha F, Zhang F, Li H (2014) Pd-catalyzed assembly of phenanthridines from aryl ketone O-acetyloximes and arynes through C-H bond activation. Tetrahedron Lett 55(5):1036–1039. https://doi.org/10.1016/j.tetlet.2013.12.075
Pimparkar S, Jeganmohan M (2014) Palladium-catalyzed cyclization of benzamides with arynes: application to the synthesis of phenaglydon and N-methylcrinasiadine. Chem Commun 50(81):12116–12119. https://doi.org/10.1039/C4CC05252H
Asamdi M, Chauhan PM, Patel JJ, Chikhalia KH (2019) Palladium catalyzed annulation of benzylamines and arynes via C-H activation to construct 5,6-dihydrophenanthridine derivatives. Tetrahedron 75(25):3485–3494. https://doi.org/10.1016/j.tet.2019.05.011
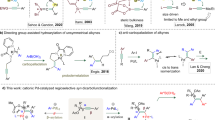
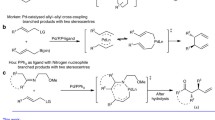
Cheng XF, Yu T, Liu Y, Wang N, Chen Z, Zhang GL, Tong L, Tang B (2022) Palladium(II)-catalyzed C(sp2)-H bond activation/C-N bond cleavage annulation of N-methoxy amides and arynes. Org Lett 24(11):2087–2092. https://doi.org/10.1021/acs.orglett.2c00161
Muzart J (2022) Cross-dehydrogenative annelation of arynes with C(sp2)-H/N-H or C(sp2)-H/O-H frameworks under Pd or Cu catalysis. Tetrahedron 126:133063. https://doi.org/10.1016/j.tet.2022.133063
Jaiswal Y, Kumar Y, Kumar A (2018) Palladium-catalyzed regioselective C-H alkenylation of arylacetamides via distal weakly coordinating primary amides as directing groups. J Org Chem 83(3):1223–1231. https://doi.org/10.1021/acs.joc.7b02618
Yang YF, Hong X, Yu JQ, Houk KN (2017) Experimental-computational synergy for selective Pd(II)-catalyzed C-H activation of aryl and alkyl groups. Acc Chem Res 50(11):2853–2860. https://doi.org/10.1021/acs.accounts.7b00440
da Silva VHM, Oliveira CC, Correia CRD, Braga AAC (2020) Heck arylation of acyclic olefins employing arenediazonium salts and chiral N, N ligands: new mechanistic insights from quantum-chemical calculations. Theor Chem Acc 139(4):77. https://doi.org/10.1007/s00214-020-02588-x
Yang YF, Cheng GJ, Liu P, Leow D, Sun TY, Chen P, Zhang X, Yu JQ, Wu YD, Houk KN (2014) Palladium-catalyzed meta-selective C-H bond activation with a nitrile-containing template: computational study on mechanism and origins of selectivity. J Am Chem Soc 136(1):344–355. https://doi.org/10.1021/ja410485g
Dutta U, Modak A, Bhaskararao B, Bera M, Bag S, Mondal A, Lupton DW, Sunoj RB, Maiti D (2017) Catalytic arene meta-C-H functionalization exploiting a quinoline-based template. ACS Catal 7(5):3162–3168. https://doi.org/10.1021/acscatal.7b00247
Musaev DG, Kaledin A, Shi BF, Yu JQ (2012) Key mechanistic features of enantioselective C-H bond activation reactions catalyzed by [(chiral mono-N-protected amino acid)-Pd(II)] complexes. J Am Chem Soc 134(3):1690–1698. https://doi.org/10.1021/ja208661v
Yao QJ, Xie PP, Wu YJ, Feng YL, Teng MY, Hong X, Shi BF (2020) Enantioselective synthesis of atropisomeric anilides via Pd(II)-catalyzed asymmetric C-H olefination. J Am Chem Soc 142(42):18266–18276. https://doi.org/10.1021/jacs.0c09400
Davies DL, Donald SMA, Macgregor SA (2005) Computational study of the mechanism of cyclometalation by palladium acetate. J Am Chem Soc 127(40):13754–13755. https://doi.org/10.1021/ja052047w
García-Cuadrado D, Braga AAC, Maseras F, Echavarren AM, (2006) Proton abstraction mechanism for the palladium-catalyzed intramolecular arylation. J Am Chem Soc 128(4):1066–1067. https://doi.org/10.1021/ja056165v
García-Cuadrado D, de Mendoza P, Braga AAC, Maseras F, Echavarren AM, (2007) Proton-abstraction mechanism in the palladium-catalyzed intramolecular arylation: substituent effects. J Am Chem Soc 129(21):6880–6886. https://doi.org/10.1021/ja071034a
Gorelsky SI, Lapointe D, Fagnou K (2008) Analysis of the concerted metalation-deprotonation mechanism in palladium-catalyzed direct arylation across a broad range of aromatic substrates. J Am Chem Soc 130(33):10848–10849. https://doi.org/10.1021/ja802533u
Lafrance M, Rowley CN, Woo TK, Fagnou K (2006) Catalytic intermolecular direct arylation of perfluorobenzenes. J Am Chem Soc 128(27):8754–8756. https://doi.org/10.1021/ja062509l
Lafrance M, Gorelsky SI, Fagnou K (2007) High-yielding palladium-catalyzed intramolecular alkane arylation: reaction development and mechanistic studies. J Am Chem Soc 129(47):14570–14571. https://doi.org/10.1021/ja076588s
Balcells D, Clot E, Eisenstein O (2010) C—H bond activation in transition metal species from a computational perspective. Chem Rev 110(2):749–823. https://doi.org/10.1021/cr900315k
Cembellín S, Dalton T, Pinkert T, Schäfers F, Glorius F, (2020) Highly selective synthesis of 1,3-enynes, pyrroles, and furans by manganese(I)-catalyzed C-H activation. ACS Catal 10(1):197–202. https://doi.org/10.1021/acscatal.9b03965
Rogge T, Oliveira JCA, Kuniyil R, Hu L, Ackermann L (2020) Reactivity-controlling factors in carboxylate-assisted C-H activation under 4d and 3d transition metal catalysis. ACS Catal 10(18):10551–10558. https://doi.org/10.1021/acscatal.0c02808
Yang Q, Wu C, Zhou J, He G, Liu H, Zhou Y (2019) Highly selective C-H bond activation of N-arylbenzimidamide and divergent couplings with diazophosphonate compounds: a catalyst-controlled selective synthetic strategy for 3-phosphorylindoles and 4-phosphorylisoquinolines. Org Chem Front 6(3):393–398. https://doi.org/10.1039/C8QO01148F
Ma W, Weng Z, Rogge T, Gu L, Lin J, Peng A, Luo X, Gou X, Ackermann L (2018) Ruthenium(II)-catalyzed C-H chalcogenation of anilides. Adv Synth Catal 360(4):704–710. https://doi.org/10.1002/adsc.201701147
Lu Q, Mondal S, Cembellín S, Glorius F, (2018) MnI/AgI relay catalysis: traceless diazo-assisted C(sp2)-H/C(sp3)-H coupling to \(\beta \)-(hetero)aryl/alkenyl ketones. Angew Chem Int Ed 57(33):10732–10736. https://doi.org/10.1002/anie.201803384
Mei R, Zhang SK, Ackermann L (2017) Ruthenium(II)-catalyzed C-H alkynylation of weakly coordinating benzoic acids. Org Lett 19(12):3171–3174. https://doi.org/10.1021/acs.orglett.7b01294
Colletto C, Islam S, Juliá-Hernández F, Larrosa I (2016) Room-temperature direct \(\beta \)-arylation of thiophenes and benzo[b]thiophenes and kinetic evidence for a heck-type pathway. J Am Chem Soc 138(5):1677–1683. https://doi.org/10.1021/jacs.5b12242
Li J, Ackermann L (2015) Cobalt(III)-catalyzed aryl and alkenyl C-H aminocarbonylation with isocyanates and acyl azides. Angew Chem Int Ed Engl 54(29):8551–8554. https://doi.org/10.1002/anie.201501926
Yu DG, Gensch T, de Azambuja F, Vásquez-Céspedes S, Glorius F (2014) Co(III)-catalyzed C-H activation/formal SN-type reactions: selective and efficient cyanation, halogenation, and allylation. J Am Chem Soc 136(51):17722–17725. https://doi.org/10.1021/ja511011m
Engle KM, Wang DH, Yu JQ (2010) Ligand-accelerated C-H activation reactions: evidence for a switch of mechanism. J Am Chem Soc 132(40):14137–14151. https://doi.org/10.1021/ja105044s
Naksomboon K, Poater J, Bickelhaupt FM, Fernández-Ibáñez MA (2019) Para-selective C-H olefination of aniline derivatives via Pd/S. O-Ligand Catalysis. J Am Chem Soc 141(16):6719–6725. https://doi.org/10.1021/jacs.9b01908
Wang L, Carrow BP (2019) Oligothiophene synthesis by a general C-H activation mechanism: electrophilic concerted metalation-deprotonation (eCMD). ACS Catal 9(8):6821–6836. https://doi.org/10.1021/acscatal.9b01195
Tan E, Quinonero O, Elena de Orbe M, Echavarren AM (2018) Broad-scope Rh-catalyzed inverse-sonogashira reaction directed by weakly coordinating groups. ACS Catal 8(3):2166–2172. https://doi.org/10.1021/acscatal.7b04395
Zell D, Bursch M, Müller V, Grimme S, Ackermann L, (2017) Full selectivity control in cobalt(III)-catalyzed C-H alkylations by switching of the C-H activation mechanism. Angew Chem Int Ed 56(35):10378–10382. https://doi.org/10.1002/anie.201704196
Ma W, Mei R, Tenti G, Ackermann L (2014) Ruthenium(II)-catalyzed oxidative C-H alkenylations of sulfonic acids, sulfonyl chlorides and sulfonamides. Chem Eur J 20(46):15248–15251. https://doi.org/10.1002/chem.201404604
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JAJ, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin K, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16, revision c. 01
Zhao Y, Truhlar DG (2006) A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J Chem Phys 125(19):194101. https://doi.org/10.1063/1.2370993
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys 7(18):3297–3305. https://doi.org/10.1039/B508541A
Weigend F (2006) Accurate coulomb-fitting basis sets for H to Rn. Phys Chem Chem Phys 8(9):1057–1065. https://doi.org/10.1039/B515623H
Gonzalez C, Schlegel HB (1989) An improved algorithm for reaction path following. J Chem Phys 90(4):2154–2161. https://doi.org/10.1063/1.456010
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113(18):6378–6396. https://doi.org/10.1021/jp810292n
Wiberg KB (1968) Application of the pople-santry-segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 24(3):1083–1096. https://doi.org/10.1016/0040-4020(68)88057-3
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88(6):899–926. https://doi.org/10.1021/cr00088a005
Cheng GJ, Yang YF, Liu P, Chen P, Sun TY, Li G, Zhang X, Houk KN, Yu JQ, Wu YD (2014) Role of N-acyl amino acid ligands in Pd(II)-catalyzed remote C-H activation of tethered arenes. J Am Chem Soc 136(3):894–897. https://doi.org/10.1021/ja411683n
Kozuch S, Shaik S (2006) A combined kinetic-quantum mechanical model for assessment of catalytic cycles: application to cross-coupling and heck reactions. J Am Chem Soc 128(10):3355–3365. https://doi.org/10.1021/ja0559146
Park CH, Ryabova V, Seregin IV, Sromek AW, Gevorgyan V (2004) Palladium-catalyzed arylation and heteroarylation of indolizines. Org Lett 6(7):1159–1162. https://doi.org/10.1021/ol049866q
Li L, Brennessel WW, Jones WD (2009) C-H activation of phenyl imines and 2-phenylpyridines with [Cp*MCl2]2 (M = Ir, Rh): regioselectivity, kinetics, and mechanism. Organometallics 28(12):3492–3500. https://doi.org/10.1021/om9000742
Pivsa-Art S, Satoh T, Kawamura Y, Miura M, Nomura M (1998) Palladium-catalyzed arylation of azole compounds with aryl halides in the presence of alkali metal carbonates and the use of copper iodide in the reaction. Bull Chem Soc Jpn 71(2):467–473. https://doi.org/10.1246/bcsj.71.467
Gorelsky SI, Lapointe D, Fagnou K (2012) Analysis of the palladium-catalyzed (aromatic)C-H bond metalation-deprotonation mechanism spanning the entire spectrum of arenes. J Org Chem 77(1):658–668. https://doi.org/10.1021/jo202342q
Jencks WP (1972) General acid-base catalysis of complex reactions in water. Chem Rev 72(6):705–718. https://doi.org/10.1021/cr60280a004
O’Ferrall RAM (1970) Relationships between E2 and E1cB mechanisms of \(\beta \)-elimination. J Chem Soc B 274–277. https://doi.org/10.1039/J29700000274
Acknowledgements
The authors acknowledge the Superintendência de Tecnologia da Informação from University of São Paulo (STI-USP), CNPq, CAPES and the São Paulo Research Foundation (FAPESP).
Funding
A.A.C.B. received financial support from São Paulo Research Foundation (FAPESP) (Grant #2015/01491-3), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) of Brazil (Grants 312550/2020-0 and 313720/2023-1), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) that partially supported this work (Finance Code 001). E.H.S.A. received financial support from FAPESP (Grant #2022/16623-6). D.A.S.O. received financial support from FAPESP (Grant #2022/01685-6) and CNPq (Grant #162366/2021-3).
Author information
Authors and Affiliations
Contributions
Erick H. S. Alves: conceptualization, methodology, performed computations, formal analysis, writing—original draft; Daniel A. S. Oliveira: conceptualization, methodology, performed computations, formal analysis, writing—original draft; Ataualpa A. C. Braga: conceptualization, methodology, formal analysis, supervision, writing-review and edition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alves, E.H.S., Oliveira, D.A.S. & Braga, A.A.C. Palladium(II)-catalyzed annulation of N-methoxy amides and arynes: computational mechanistic insights and substituents effects. J Mol Model 30, 152 (2024). https://doi.org/10.1007/s00894-024-05930-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-024-05930-3