Abstract
Context
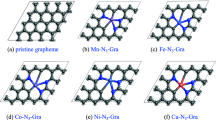
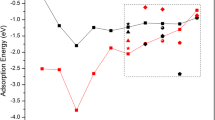
Finding catalysts that do not rely on the use of expensive metals is one of the requirements to achieve sustainable production. The reactivity of graphene doped with 3d transition metals was studied. All dopants enhanced the reactivity of graphene and performed better than Stone–Wales defects and divacancies, but were inferior to monovacancies. For hydrogenation of doped-monovacancies, Sc, Ti, Cr, Co, and Ni induced more prominent reactivity on the carbon atoms. However, the metals were the most reactive center for V, Mn, and Fe-doped graphene. Cu and Zn turned the four neighboring carbon atoms into the preferred sites for hydrogenation. The addition of oxygen to doped graphene with Ti, V, Cr, Mn, Fe, Co, and Ni on a monovacancy revealed a more uniform pattern since the metal, preferred to react with oxygen. However, Sc induced a larger reactivity on the carbon atoms. The affinity of the 3d metal-doped graphene systems towards oxygen was inferior to that observed for single-vacancies. Therefore, vacancy engineering is the most favorable and least expensive method to enhance the reactivity of graphene.
Methods
We applied Truhlar’s M06-L method accompanied by the 6-31G* basis sets to perform periodic boundary conditions calculations as implemented in Gaussian 09. The ultrafine grid was employed and the unit cells were sampled employing 100 k-points. Results were visualized employing Gaussview 5.0.1





Similar content being viewed by others
Data availability
Data is available upon request.
References
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Frisov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669
Novoselov KS, Geim AK, Morozov SV, Jiang D, Katsnelson MI, Grigorieva IV, Dubonos SV, Frisov AA (2005) Two-dimensional gas of massless Dirac fermions in graphene. Nature 438:197–200
Panchakarla LS, Subrahmanyam KS, Saha SK, Govindaraj A, Krishnamurthy HR, Waghmare UV, Rao CNR (2009) Synthesis, structure, and properties of boron- and nitrogen-doped graphene. Adv Mater 21:4726–4730
Wang L, Sofer Z, Simek P, Tomandl I, Pumera M (2013) Boron-doped graphene: scalable and tunable p-type carrier concentration doping. J Phys Chem C 117:23251–23257
Laref A, Ahmed A, Binomran S, Luo SJ (2015) First-principle analysis of the electronic and optical properties of boron and nitrogen doped carbon mono-layer graphenes. Carbon 81:179–192
Lv R, Li Q, Botello-Mendez AR, Hayashi T, Wang B, Berkdemir A, Hao Q, Elias AL, Cruz-Silva E, Gutierrez HR, Kim YA, Muramatsu H, Zhu J, Endo M, Terrones H, Charlier JC, Pan M, Terrones M (2012) Nitrogen-doped graphene: beyond single substitution and enhanced molecular sensing. Sci Rep 2:586
Yuan Z, Li J, Yang M, Fang Z, Jian J, Yu D, Chen X, Dai L (2019) Ultrathin black phosphorus-on-nitrogen doped graphene for efficient overall water splitting: dual modulation roles of directional interfacial charge transfer. J Am Chem Soc 12:4972–4979
Ullah S, Denis PA, Sato F (2019) Coupled cluster investigation of the interaction of beryllium, magnesium, and calcium with pyridine: implications for the adsorption on nitrogen-doped graphene. Comput Theor Chem 1150:57–62
Ullah S, Hussain A, Syed W, Saqlain MA, Ahmad I, Leenaerts O, Karim A (2015) Band-gap tuning of graphene by Be doping and Be, B co-doping: a DFT study. RSC Adv 5:55762–55773
Ullah S, Denis PA, Sato F (2017) Beryllium doped graphene as an efficient anode material for lithium-ion batteries with significantly huge capacity: a DFT study. Appl Mater Today 9:333–340
Ullah S, Liu Y, Hasan M, Zeng W, Shi Q, Yang X, Fu L, Ta HQ, Lian X, Sun J, Yang R, Liu L, Rümmeli MH (2022) Direct synthesis of large-area Al-doped graphene by chemical vapor deposition: advancing the substitutionally doped graphene family. Nano Res 15:1310–1318
Zagler G, Stecher M, Trentino A, Kraft F, Su C, Post A, Längle M, Pesenhofer C, Mangler C, Åhlgren EH, Markevich A, Zettl A, Kotakoski J, Susi T, Mustonen K (2022) Beam-driven dynamics of aluminium dopants in graphene. 2D Mater 9:035009
Denis PA (2011) Tuning the electronic properties of doped bilayer graphene with small structural changes. Comput Theor Chem 974:21–25
Tayyab M, Hussain A, Adil W, Nabi S, Ul Ain Asif Q (2020) Band-gap engineering of graphene by Al doping and adsorption of Be and Br on impurity: a computational study. Comput Cond Matter 23:e00463
Denis PA (2010) Band gap opening of monolayer and bilayer graphene doped with aluminium, silicon, phosphorus, and sulfur. Chem Phys Lett 492:251–257
Zhou W, Kapetanakis MD, Prange MP, Pantelides ST, Pennycook SJ, Idrobo JC (2012) Direct determination of the chemical bonding of individual impurities in graphene. Phys Rev Lett 109:206803
Denis PA (2016) Heteroatom promoted cycloadditions for graphene. ChemistrySelect 1:5497–5500
Ramasse QM, Seabourne CR, Kepaptsoglou DM, Zan R, Bangert U, Scott AJ (2013) Probing the bonding and electronic structure of single atom dopants in graphene with electron energy loss spectroscopy. Nano Lett 13:4989–4995
Rafique M, Shuai Y, Hussain N (2018) First-principles study on silicon atom doped monolayer graphene. Phys E: Low-Dimens Syst Nanostructures 95:94–101
Houmad M, Zaari H, Benyoussef A, El Kenz A, Ez-Zahraouy H (2015) Optical conductivity enhancement and band gap opening with silicon doped graphene. Carbon 94:1021–1027
Denis PA (2011) When noncovalent interactions are stronger than covalent bonds: Bilayer graphene doped with second row atoms, aluminum, silicon, phosphorus and sulfur. Chem Phys Lett 508:95–101
Some S, Kim J, Lee K, Kulkarni A, Yoon Y, Lee S, Kim T, Lee H (2012) Highly air-stable phosphorus-doped n-type graphene field-effect transistors. Adv Mater 24:5481–5486
Li R, Wei Z, Gou X, Xu W (2013) Phosphorus-doped graphene nanosheets as efficient metal-free oxygen reduction electrocatalysts. RSC Adv 3:9978–9984
Zhang C, Mahmood N, Yin H, Liu F (2013) Synthesis of phosphorus-doped graphene and its multifunctional applications for oxygen reduction reaction and lithium ion batteries. Adv Mater 25:4932–4937
Niu F, Tao LM, Deng YC, Wang QH (2014) Phosphorus doped graphene nanosheets for room temperature NH3 sensing. New J Chem 38:2269–2272
Susi T, Hardcastle TP, Hofsäss H, Mittelberger A, Pennycook TJ, Mangler C, Drummond-Brydson R, Scott AJ, Meyer JC, Kotakoski J (2017) Single-atom spectroscopy of phosphorus dopants implanted into graphene. 2D Mater 4:021013
Gonzalez Larrude D, Garcia-Basabe Y, LazaroFreireJr F, Rocco MLM (2015) Electronic structure and ultrafast charge transfer dynamics of phosphorous doped graphene layers on a copper substrate: a combined spectroscopic study. RSC Adv 5:74189–74197
Denis PA (2013) Concentration dependence of the band gaps of phosphorus and sulfur doped graphene. Comput Mater Sci 67:203–206
Denis PA, Faccio R, Mombru AW (2009) Is it possible to dope single-walled carbon nanotubes and graphene with sulfur? ChemPhysChem 10:715–722
Yang Z, Yao Z, Li G, Fang G, Nie H, Liu Z, Zhou X, Chen X, Huang S (2012) Sulfur-doped graphene as an efficient metal-free cathode catalyst for oxygen reduction. ACS Nano 6:205–211
Yang S, Zhi L, Tang K, Feng X, Maier J, Müllen K (2012) Efficient synthesis of heteroatom (N or S)-doped graphene based on ultrathin graphene oxide-porous silica sheets for oxygen reduction reactions. Adv Funct Mater 22:3634–3640
Rao A, Quan W, Vajtai R, Ajayan PM (2012) Synthesis of S-doped graphene by liquid precursor. Nanotechnology 23:275605
Zhang J, Li J, Wang Z, Wang X, Feng W, Zheng W, Cao W, Hu P (2014) Low-Temperature growth of large-area heteroatom-doped graphene film. Chem Mater 26:2460–2466
Wang Z, Li P, Chen Y, He J, Zhang W, Schmidt OG, Li Y (2014) Pure thiophene–sulfur doped reduced graphene oxide: synthesis, structure, and electrical properties. Nanoscale 6:7281–7287
Poh HL, Šimek P, Sofer Z, Pumera M (2013) Sulfur-doped graphene via thermal exfoliation of graphite oxide in H2S, SO2, or CS2 gas. ACS Nano 7:5262–5272
Tripathi M, Markevich A, Böttger R, Facsko S, Besley E, Kotakoski J, Susi T (2018) Implanting germanium into graphene. ACS Nano 12:4641–4647
Meng X, Yu C, Song X, Iocozzia J, Hong J, Rager M, Jin H, Wang S, Huang L, Qiu J, Lin Z (2018) Scrutinizing defects and defect density of selenium-doped graphene for high-efficiency triiodide reduction in dye-sensitized solar cells. Angew Chem Int Ed 57:4772–4682
Denis PA (2014) Chemical reactivity and band-gap opening of graphene doped with gallium, germanium, arsenic, and selenium atoms. ChemPhysChem 15:3994–4000
Krasheninnikov AV, Lehtinen PO, Foster AS, Pyykkö P, Nieminen RM (2009) Embedding transition-metal atoms in graphene: structure, bonding, and magnetism. Phys Rev Lett 102:126807
Toh RJ, Poh HL, Sofer Z, Pumera M (2013) Transition metal (Mn, Fe Co, Ni)-doped graphene hybrids for electrocatalysis. Chem Asian J 8:1295–1300
Robertson AW, Montanari B, He K (2013) J. Kim, C.S. Allen, Y.A. Wu, J. Olivier, J. Neethling, N. Harrison, A.I. Kirkland, J.H. Warner, Dynamics of single fe atoms in graphene vacancies. Nano Lett 13:1468–1475
Zhao J, Deng Q, Bachmatiuk A, Sandeep G, Popov A, Eckert J, Rümmeli MH (2014) Free-standing single-atom-thick iron membranes suspended in graphene pores. Science 343:1228–1232
Lin PC, Villarreal R, Achilli S, Bana H, Nair MN, Tejeda A, Verguts K, De Gendt S, Auge M, Hofsäss H, De Feyter S, Di Santo G, Petaccia L, Brems S, Fratesi G, Pereira LMC (2015) Doping graphene with substitutional Mn. ACS Nano 15:5449–5458
Carnevali V, Patera LL, Prandini G, Jugovac M, Modesti S, Comelli G, Peressi M, Africh C (2019) Doping of epitaxial graphene by direct incorporation of nickel adatoms. Nanoscale 11:10358–10364
Wen X, Duan Z, Bai L, Guan J (2019) Atomic scandium and nitrogen-codoped graphene for oxygen reduction reaction. J Power Sources 431:265–273
Zitolo A, Goellner V, Armel V, Sougrati MT, Mineva T, Stievano L, Fonda E, Jaouen F (2015) Identification of catalytic sites for oxygen reduction in iron-and nitrogen-doped graphene materials. Nat Mater 14:937–942
Niu Y, Huang X, Hu W (2016) Fe3C nanoparticle decorated Fe/N doped graphene for efficient oxygen reduction reaction electrocatalysis. J Power Sources 332:305–311
Ni Y, Chen Z, Kong F, Qiao Y, Kong A, Shan Y (2017) Space-confined synthesis of multilayer Cu–N-doped graphene nanosheets for efficient oxygen electroreduction. Dalton Trans 46:8586–8592
Zhu G, Liu F, Wang Y, Wei Z, Wang W (2019) Systematic exploration of N, C coordination effects on the ORR performance of Mn–Nx doped graphene catalysts based on DFT calculations. Phys Chem Chem Phys 21:12826–12836
Luo M, Liang Z, Liu C, Qi X, Chen M, Sagar RUR, Yang H, Liang T (2021) Single–atom manganese and nitrogen co-doped graphene as low-cost catalysts for the efficient CO oxidation at room temperature. Appl Surf Sci 536:147809
Du Z, Chen X, Hu W, Chuang C, Xie S, Hu A, Yan W, Kong X, Wu X, Ji H, Wan LJ (2019) Cobalt in nitrogen-doped graphene as single-atom catalyst for high-sulfur content lithium–sulfur batteries. J Am Chem Soc 141:3977–3985
Trentino A, Mizohata K, Zagler G, Längle M, Mustonen K, Susi T, Kotakoski J, Åhlgren EH (2022) Two-step implantation of gold into graphene. 2D Mater 9:025011
Denis PA, Iribarne F (2013) Comparative study of defect reactivity in graphene. J Phys Chem C 117:5612–5619
Denis PA, Huelmo CP (2015) Structural characterization and chemical reactivity of dual doped graphene. Carbon 87:106–115
Denis PA, Huelmo CP, Iribarne F (2014) Theoretical characterization of sulfur and nitrogen dual-doped graphene. Comp Theor Chem 1049:13–19
Denis PA, Iribarne F (2016) The effect of the dopant nature on the reactivity, interlayer bonding and electronic properties of dual doped bilayer graphene. Phys Chem Chem Phys 18:24693–24703
Ullah S, Denis PA, Sato F (2017) Triple-doped monolayer graphene with boron, nitrogen, aluminum, silicon, phosphorus, and sulfur. ChemPhysChem 18:1864–1873
Ali S, Liu T, Li B, Su DS (2017) The tunable effect of nitrogen and boron dopants on a single walled carbon nanotube support on the catalytic properties of a single gold atom catalyst: a first principles study of CO oxidation. J Mater Chem A 5:16653–16662
Ali S, Lian Z, Li B (2021) Density Functional theory study of a graphdiyne-supported single au atom catalyst for highly efficient acetylene hydrochlorination. ACS Appl Nano Mater 4:6893–6902
Ali S, Olanrele S, Liu T, Si C, Yang M, Li B (2019) Single Au anion can catalyze acetylene hydrochlorination: tunable catalytic performance from rational doping. J Phys Chem C 123:29203–29208
Ali S, Liu T, Li B, Su DS, Li B (2019) The stability and reactivity of transition metal atoms supported mono and di vacancies defected carbon based materials revealed from first principles study. Appl Surf Sci 473:777–784
Ali S, Iqbal R, Kahn A, Rehman SU, Haneef M, Yin L (2021) Stability and catalytic performance of single-atom catalysts supported on doped and defective graphene for CO2 hydrogenation to formic acid: a first-principles study. ACS Appl Nano Mater 4:6893–6902
Ismail PM, Ali S, Raziq S, Bououdina M, Abu-Farsakh H, Xia P, Wu X, Xiao H, Ali S, Qiao L (2023) Stable and robust single transition metal atom catalyst for CO2 reduction supported on defective WS2. Appl Surf Sci 624:157073
Ali A, Xie Z, Xu H (2021) Stability and catalytic performance of single-atom supported on Ti2CO2 for low-temperature CO oxidation: a first-principles study. ChemPhysChem 22:2352
Ali S, Yasin G, Iqbal R, Huang X, Su J, Ibraheem S, Zhang Z, Wu X, Wahid F, Ismail PM, Qiao L, Xu H (2022) Porous aza-doped graphene-analogous 2D material a unique catalyst for CO2 conversion to formic-acid by hydrogenation and electroreduction approaches. Mol Catal 524:112285
Yasin G, Ali S, Ibraheem S, Kumar A, Tabish M, Mushtaq MA, Ajmal S, Arif M, Kham MA, Saad A, Qiao L, Zhao W (2023) Simultaneously engineering the synergistic-effects and coordination-environment of dual-single-atomic iron/cobalt-sites as a bifunctional oxygen electrocatalyst for rechargeable zinc-air batteries. ACS Catal 13:2313–2325
Ali S, Haneef M, Akbar J, Ullah I, Ullah S, Samad A (2021) Single Au atom supported defect mediated boron nitride monolayer as an efficient catalyst for acetylene hydrochlorination: A first principles study. Mol Catal 511:111753
Zhao Y, Truhlar DG (2006) New local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J Chem Phys 125:194101
Gaussian 09, Revision D.01, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox, Gaussian, Inc., Wallingford CT, 2009.
Hehre W, Radom L, Schleyer PvR, Pople JA (1986) Ab initio molecular orbital theory. Wiley, New York
Chen X, Xu L, Liu LL, Zhao LS, Chen CP, Zhang Y, Wang XC (2017) Adsorption of formaldehyde molecule on the pristine and transition metal doped graphene: first-principles study. Appl Surf Sci 396:1020–1025
Acknowledgements
The author thanks PEDECIBA−Quimica, ANII and CSIC for financial support.
Author information
Authors and Affiliations
Contributions
The manuscript has one author who made the contributions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Denis, P.A. Chemical reactivity of graphene doped with 3d transition metals: nothing compares to a single vacancy. J Mol Model 30, 96 (2024). https://doi.org/10.1007/s00894-024-05893-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-024-05893-5