Abstract
Context
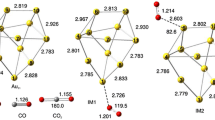
The increasing demand for fuels and chemicals in the world has prompted the exploration of various forms of renewable energy resources. Using C5-based furfural as the platform to replace the fossil energy resources is greatly attractive because of its abundance and environmental friendliness. Here we study the activity, selectivity, and possible reaction pathways for the Baeyer–Villiger oxidation of furfural over small Au clusters using hydrogen peroxide as oxidant. Furfural reacts with hydrogen peroxide in the presence of the catalysts with 93% selectivity towards maleic anhydride. Natural population analysis, frontier molecular orbital analysis, and spectroscopic analysis are used to illustrate the interaction mechanism between C5H4O2, H2O2, and Au. Reaction pathways leading to the formation of maleic anhydride are also explored. The reaction of C5H4O2 with H2O2 in the absence of a catalyst bears a relatively high transition state energy barrier of 2.98 eV for the first step involving absorption of H atom of H2O2 on the –OH group of C5H4O2. This is in agreement with the blank experiment where there were rare oxidation products observed in the absence of the metal cluster catalysts. On the other hand, transition state energies in the presence of the Au metal clusters are lower and the most feasible pathway is where the substrate and H2O2 co-bind on the Au catalyst and H2O2 molecule transfers an oxygen to the substrate, leading to the cleavage of the O–O bond.
Methods
DFT calculations were done with B3PW91 functional. 6-311G(df, p) basis set was used for C, O, and H and aug-cc-pVDZ-PP was used for gold atoms. Gaussian 09 software was used for the calculations. Multiwfn 3.7 dev was used for the quantum theory of atoms-in-molecules (QTAIM) investigations.










Similar content being viewed by others
Data availability
Not applicable.
References
Liu S, Amada Y, Tamura M, Nakagawa Y, Tomishige K (2014) One-pot selective conversion of furfural into 1, 5-pentanediol over a Pd-added Ir–Reo X/Sio 2 bifunctional catalyst. Green Chem 16:617–626
Alonso DM, Bond JQ, Dumesic JA (2010) Catalytic conversion of biomass to biofuels. Green Chem 12:1493–1513
Tachibana Y, Masuda T, Funabashi M, Kunioka M (2010) Chemical synthesis of fully biomass-based poly (butylene succinate) from inedible-biomass-based furfural and evaluation of its biomass carbon ratio. Biomacromol 11:2760–2765
Qiao Y, Theyssen N, Hou Z (2015) Acid-catalyzed dehydration of fructose to 5-(hydroxymethyl) furfural. Recyclable Catalysis 2:36–60
Kwon Y, de Jong E, Raoufmoghaddam S, Koper M (2013) Electrocatalytic hydrogenation of 5-hydroxymethylfurfural in the absence and presence of glucose. Chemsuschem 6:1659–1667
Du Z, Ma J, Wang F, Liu J, Xu J (2011) Oxidation of 5-hydroxymethylfurfural to maleic anhydride with molecular oxygen. Green Chem 13:554–557
Yan N, Zhao C, Luo C, Dyson PJ, Liu H, Kou Y (2006) One-step conversion of cellobiose to C6-alcohols using a ruthenium nanocluster catalyst. J Am Chem Soc 128:8714–8715
Lan J, Chen Z, Lin J, Yin G (2014) Catalytic aerobic oxidation of renewable furfural to maleic anhydride and furanone derivatives with their mechanistic studies. Green Chem 16:4351–4358
Xu W, Xia Q, Zhang Y, Guo Y, Wang Y, Lu G (2011) Effective production of octane from biomass derivatives under mild conditions. Chemsuschem 4:1758–1761
Xu W, Wang H, Liu X, Ren J, Wang Y, Lu G (2011) Direct catalytic conversion of furfural to 1, 5-pentanediol by hydrogenolysis of the furan ring under mild conditions over Pt/Co 2 Alo 4 catalyst. Chem Commun 47:3924–3926
Serrano-Ruiz JC, Luque R, Sepulveda-Escribano A (2011) Transformations of biomass-derived platform molecules: from high added-value chemicals to fuels via aqueous-phase processing. Chem Soc Rev 40:5266–5281
Menegazzo F, Signoretto M, Pinna F, Manzoli M, Aina V, Cerrato G, Boccuzzi F (2014) Oxidative esterification of renewable furfural on gold-based catalysts: which is the best support? J Catal 309:241–247
Baeyer A, Villiger V (1899) Einwirkung Des Caro’schen Reagens Auf Ketone. Eur J Inorg Chem 32:3625–3633
Uyanik M, Ishihara K (2013) Baeyer-Villiger oxidation using hydrogen peroxide. ACS Catal 3:513–520
Alonso-Fagúndez N, Agirrezabal-Telleria I, Arias P, Fierro J, Mariscal R, Granados ML (2014) Aqueous-phase catalytic oxidation of furfural with H 2 O 2: high yield of maleic acid by using titanium silicalite-1. RSC Adv 4:54960–54972
Choudhary H, Nishimura S, Ebitani K (2012) Highly efficient aqueous oxidation of furfural to succinic acid using reusable heterogeneous acid catalyst with hydrogen peroxide. Chem Lett 41:409–411
Alonso-Fagúndez N, Granados ML, Mariscal R, Ojeda M (2012) Selective conversion of furfural to maleic anhydride and furan with Vox/Al2o3 catalysts. Chemsuschem 5:1984–1990
Lohbeck K, Haferkorn H, Fuhrmann W, Fedtke N (2000) Maleic and fumaric acids. Ullmann’s encyclopedia of industrial chemistry
Luo Z, Castleman Jr A, Khanna SN (2016) Reactivity of metal clusters. Chem Rev
Heck RM, Farrauto RJ (2001) Automobile exhaust catalysts. Appl Catal A 221:443–457
Bell AT (2003) The impact of nanoscience on heterogeneous catalysis. Science 299:1688–1691
Deluga G, Salge J, Schmidt L, Verykios X (2004) Renewable hydrogen from ethanol by autothermal reforming. Science 303:993–997
Chen M, Goodman D (2004) The structure of catalytically active gold on titania. Science 306:252–255
Zhang J, Sasaki K, Sutter E, Adzic R (2007) Stabilization of platinum oxygen-reduction electrocatalysts using gold clusters. Science 315:220–222
Antolini E (2009) Carbon supports for low-temperature fuel cell catalysts. Appl Catal B 88:1–24
Ma Z, Dai S (2011) Design of novel structured gold nanocatalysts. ACS Catal 1:805–818
Liu J (2016) Catalysis by supported single metal atoms. ACS Catal 7:34–59
Gates B (1995) Supported metal clusters: synthesis, structure, and catalysis. Chem Rev 95:511–522
Lopez N, Janssens T, Clausen B, Xu Y, Mavrikakis M, Bligaard T, Nørskov JK (2004) On the origin of the catalytic activity of gold nanoparticles for low-temperature Co oxidation. J Catal 223:232–235
Kubo R (1962) Electronic properties of metallic fine particles. I. J Phys Soc Jpn 17:975–986
Issendorff BV, Cheshnovsky O (2005) Metal to insulator transitions in clusters. Annu Rev Phys Chem 56:549–580
Claus P, Brückner A, Mohr C, Hofmeister H (2000) Supported gold nanoparticles from quantum dot to mesoscopic size scale: effect of electronic and structural properties on catalytic hydrogenation of conjugated functional groups. J Am Chem Soc 122:11430–11439
Haruta M (1997) Size-and support-dependency in the catalysis of gold. Catal Today 36:153–166
Haruta M, Kobayashi T, Sano H, Yamada N (1987) Novel gold catalysts for the oxidation of carbon monoxide at a temperature far below 0 C. Chem Lett 16:405–408
Xu Z, Xiao F-S, Purnell S, Alexeev O, Kawi S, Deutsch S, Gates B (1994) Size-dependent catalytic activity of supported metal clusters. Nature 372:346–348
Pembere A, Luo Z (2017) Jones oxidation of glycerol catalyzed by small gold clusters. Phys Chem Chem Phys. https://doi.org/10.1039/C6CP07941E
Nijhuis TA, Visser T, Weckhuysen BM (2005) Mechanistic study into the direct epoxidation of propene over gold/titania catalysts. J Phys Chem B 109:19309–19319
Tsunoyama H, Sakurai H, Ichikuni N, Negishi Y, Tsukuda T (2004) Colloidal gold nanoparticles as catalyst for carbon− carbon bond formation: application to aerobic homocoupling of phenylboronic acid in water. Langmuir 20:11293–11296
Boorman TC, Larrosa I (2011) Gold-mediated C-H bond functionalisation. Chem Soc Rev 40:1910–1925
McEwan L, Julius M, Roberts S, Fletcher JC (2010) A review of the use of gold catalysts in selective hydrogenation reactions. Gold Bull 43
Bond G (2009) Mechanisms of the gold-catalysed water-gas shift. Gold Bull 42:337–342
Rajesh R, Sujanthi E, Kumar SS, Venkatesan R (2015) Designing versatile heterogeneous catalysts based on Ag and Au nanoparticles decorated on chitosan functionalized graphene oxide. Phys Chem Chem Phys 17:11329–11340
Cao HL, Huang HB, Chen Z, Karadeniz B, Lu J, Cao R (2017) Ultrafine silver nanoparticles supported on a conjugated microporous polymer as high-performance nanocatalysts for nitrophenol reduction. ACS Appl Mater Interfaces 9:5231–5236
Molina LM et al (2011) Size-dependent selectivity and activity of silver nanoclusters in the partial oxidation of propylene to propylene oxide and acrolein: a joint experimental and theoretical study. Catal Today 160:116–130
Anthony M.S. Pembere, Hitler Louis, Haiming Wu (2023) Probing the interaction of Ti clusters with isopropanol for ether production: an experimental and computational study Transition Metal Chemistry volume 48, pages227–235
Pembere AM, Cui C, Wu H, Luo Z (2019) Small gold clusters catalyzing oxidant-free dehydrogenation of glycerol initiated by methene hydrogen atom transfer. Chin Chem Lett 30:1000–1004
Pembere AM, Yang M, Luo Z (2017) Small gold clusters catalyzing the conversion of glycerol to epichlorohydrin. Phys Chem Chem Phys 19:25840–25845
Pembere AM, Wu H, An P, Magero D, Louis H, Luo Z (2022) Guerbet coupling of methanol catalysed by titanium clusters. Chem Phys Lett 139719
Burke K, Perdew JP, Wang Y (1998) Derivation of a generalized gradient approximation: the Pw91 density functional. Springer, In Electronic density functional theory, pp 81–111
Lyalin A, Taketsugu T (2010) Reactant-promoted oxygen dissociation on gold clusters. J Phys Chem Lett 1:1752–1757
Lyalin A, Taketsugu T (2009) Cooperative adsorption of O2 and C2h4 on small gold clusters. J Phys Chem C 113:12930–12934
Lyalin A, Taketsugu T (2010) Adsorption of ethylene on neutral, anionic, and cationic gold clusters. J Phys Chem C 114:2484–2493
Li J, Li X, Zhai H-J, Wang L-S (2003) Au20: a tetrahedral cluster. Science 299:864–867
Idrobo JC, Walkosz W, Yip SF, Öğüt S, Wang J, Jellinek J (2007) Static polarizabilities and optical absorption spectra of gold clusters (Au N, N= 2–14 and 20) from first principles. Phys Rev B 76:205422
Xiao L, Tollberg B, Hu X, Wang L (2006) Structural study of gold clusters. J Chem Phys 124:114309
Dill JD, Pople JA (1975) Self-consistent molecular orbital methods. Xv. Extended Gaussian-type basis sets for lithium, beryllium, and boron. J Chem Phys 62:2921–2923
Pyykkö P (2004) Theoretical chemistry of gold. Angew Chem Int Ed 43:4412–4456
Figgen D, Peterson KA, Dolg M, Stoll H (2009) Energy-consistent pseudopotentials and correlation consistent basis sets for the 5 D elements Hf–Pt. J Chem Phys 130:164108
Peterson KA, Puzzarini C (2005) Systematically convergent basis sets for transition metals. Ii. Pseudopotential-based correlation consistent basis sets for the group 11 (Cu, Ag, Au) and 12 (Zn, Cd, Hg) elements. Theor Chem Acc 114:283–296
Miertus S, Tomasi J (1982) Approximate evaluations of the electrostatic free energy and internal energy changes in solution processes. Chem Phys 65:239–245
Glendening E, Reed A, Carpenter J, Weinhold F (1998) Nbo Version 3.1, Tci. University of Wisconsin, Madison 65
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Li X, Jia P, Wang T (2016) Furfural: a promising platform compound for sustainable production of C4 and C5 chemicals. ACS Catal 6:7621–7640
Xie Y, Huang Y, Wu C, Yuan W, Xia Y, Liu X, Wang H (2018) Iron-based metalloporphyrins as efficient catalysts for aerobic oxidation of biomass derived furfural into maleic acid Mol. Catal 452:20–27
Huang Y, Wu C, Yuan W, Xia Y, Liu X, Yang H, Wang H (2017) Catalytic aerobic oxidation of biomass-based furfural into maleic acid in aqueous phase with metalloporphyrin catalysts. J Chin Chem Soc 64:786–794
Lv G, Chen C, Lu B, Li J, Yang Y, Chen C, Deng T, Zhu Y, Hou X (2016) Vanadium-oxo immobilized onto Schiff base modified graphene oxide for efficient catalytic oxidation of 5-hydroxymethylfurfural and furfural into maleic anhydride. RSC Adv 6:101277–101282
Sotak T, Hronec M, Gal M, Dobrocka E, Skriniarova J (2017) Aqueous-phase oxidation of furfural to maleic acid catalyzed by copper phosphate catalysts Catal. Lett 147:2714–2723
Lou Y, Marinkovic S, Estrine B, Qiang W, Enderlin G (2020) Oxidation of furfural and furan derivatives to maleic acid in the presence of a simple catalyst system based on acetic acid and TS-1 and hydrogen peroxide. ACS Omega 5:2561–2568
Nguyen CV, Boo JR, Liu C-H, Ahamad T, Alshehri SM, Matsagar BM, Wu KCW (2020) Catal. Sci Technol 10:1498–1506
Yu Q, Bai R, Wang F, Zhang Q, Sun Y, Zhang Y, Qin L, Wang Z, Yuan Z (2020) Oxidation of biomass-derived furans to maleic acid over nitrogen-doped carbon catalysts under acid-free conditions. J Chem Technol Biotechnol 95:751–757
Zhang H, Wang S, Zhang H, Clark JH, Cao F (2021) A biomass-derived metal-free catalyst doped with phosphorus for highly efficient and selective oxidation of furfural into maleic acid. Green Chem 23:1370–1381
Yang T, Li W, Liu Q, Su M, Zhang T, Ma J (2019) Synthesis of maleic acid from biomass-derived furfural in the presence of KBr/graphitic carbon nitride (gC 3 N 4) catalyst and hydrogen peroxide. Bioresour 14:5025–5044
Yang T, Li W, Ogunbiyi AT (2021) Recent advances in the conversion of furfural into bio-chemicals through chemo-and bio-catalysisMol. Catal 504:111488
Malibo PM, Makgwane PR, Baker PG (2020) Heterostructured redox-active V2O5/SnO2 oxide nanocatalyst for aqueous-phase oxidation of furfural to renewable maleic acid. ChemistrySelect 5:6255–6267
Zhao X, Kong X, Wang F, Fang R, Li Y (2021) Metal sub-nanoclusters confined within hierarchical porous carbons with high oxidation activity. Angew Chem Int Ed 60:10842–10849
Vorotnikov V, Mpourmpakis G, Vlachos DG (2012) Dft study of furfural conversion to furan, furfuryl alcohol, and 2-methylfuran on Pd (111). ACS Catal 2:2496–2504
Bhogeswararao S, Srinivas D (2015) Catalytic conversion of furfural to industrial chemicals over supported Pt and Pd catalysts. J Catal 327:65–77
Shekhar R, Barteau MA, Plank RV, Vohs JM (1997) Adsorption and reaction of aldehydes on Pd surfaces. J Phys Chem B 101:7939–7951
Bader RFJAOCR (1985) Atoms in molecules 18:9–15
Funding
This work was also financially supported by the Key Research Program of Frontier Sciences (CAS, Grant QYZDB-SSW-SLH024) and the National Natural Science Foundation of China (Grant No. 21722308). Theoretical calculations were done using the facilities at the Center for High Performance Computing, South Africa.
Author information
Authors and Affiliations
Contributions
A.P: performing experiments and drafting the manuscript.
H.L: theoretical calculations.
H.W: reviewing the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pembere, A.M.S., Louis, H. & Wu, H. Mechanism and dynamics of Baeyer–Villiger oxidation of furfural to maleic anhydride in presence of H2O2 and Au clusters. J Mol Model 29, 359 (2023). https://doi.org/10.1007/s00894-023-05764-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05764-5