Abstract
Context
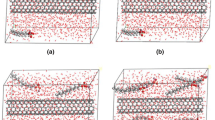
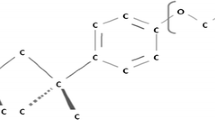
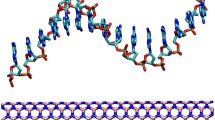
The aggregation and adsorption of amantadine and sodium dodecyl sulfate on the boron nitride nanotubes in aqueous system were investigated, employing the classical molecular dynamics method. The aqueous system containing amantadine and sodium dodecyl sulfate was investigated by self-diffusion of the molecules, with particular emphasis on their center of mass mean square displacement. The radial distribution function and dipole moment measurement were used to analyze the water effect on the aggregation and molecular interaction between amantadine and sodium dodecyl sulfate in the complex. In turn, the amantadine molecules are able to develop water monolayers at the aqueous solution but show no noticeable aggregated adsorption in the fluid. In contrast, sodium dodecyl sulfate self-aggregation on the boron nitride nanotube efficiently occurs, and so the effect of sodium dodecyl sulfate on aggregated adsorption of amantadine was studied. Our results show that in the presence of sodium dodecyl sulfate at critical micelle concentration, its effect on the aggregation of amantadine was enhanced onto boron nitride nanotube compared to just pure sodium dodecyl sulfate or amantadine on boron nitride nanotube. Our simulations show a remarkable restructuring and enhanced orientation of the water molecules around sodium dodecyl sulfate and amantadine adsorbed on boron nitride nanotube.
Method
All molecular dynamics simulations were done using NAMD-2.9 package with CHARMM-36 force field. The nanotube PDB file was made by VMD with (12, 12) indexes. The nanotube atoms were considered to be fixed and periodic during the simulation process, and the ends did not cap with hydrogens. The initial PDB file of components was saved from ChemOffice software, and the webservers to obtain the charge and topology files were Reddb and Swiss Param webservers. The VMD software was used for structural visualizations and graphical analysis.











Similar content being viewed by others
Data Availability
The calculated data can be accessed.
References
Wei Y, Wang H, Liu G, Wang Z, Yuan S (2016) A molecular dynamics study on two promising green surfactant micelles of choline dodecyl sulfate and laurate. RSC Adv 6:84090
Yang W, Wang T, Fan Z, Miao Q, Deng Z, Zhu Y (2017) Foams stabilized by in sit modified nanoparticles and anionic surfactants for enhanced oil recovery. Energy Fuel 31:4721–4730
Sun Q, Li Z, Li S, Jiang L, Wang J, Wang P (2014) Utilization of surfactant-stabilized foam for enhanced oil recovery by adding nanoparticles. Energy Fuel 28:2384–2394
Guo F, Aryana S (2016) an experimental investigation of nanoparticle-stabilized CO2 foam used in enhanced oil recovery. Journal of Fuels 186:430–442
Sun S, Zhang X, Wang P, Wang H, Wang Z, Luo J, Li C, Hu S (2019) Emulsified oil phase induced internal instability of ionic and nonionic foams revealed by coarse-grained molecular dynamics simulation. Comput Mater Sci 169:109111–109119
Bera A, Mandal A (2015) Microemulsions: a novel approach to enhanced oil recovery: a review. J Pet Explor Prod Technol 5:255–268
Fameau AL, Arnould A, Saint-Jalmes A (2014) Responsive self-assemblies based on fatty acids. Curr Opin Colloid Interface Sci 19:471–479
Rodríguez Patino JM, Carrera Sánchez C, Nino MRR (2008) Implications of interfacial characteristics of food foaming agents in foam formulations. Adv Colloid Interface Sci 140(2):95–113
Chen M, Lu X, Liu X, Hou Q, Zhu Y, Zhu H (2014) Specific counterion effects on the atomistic structure and capillary-waves fluctuation of the water/vapor interface covered by sodium dodecyl sulfate. J Phys Chem C 118:19205–19213
Gao S, Kang Z, Yuan R, Liu N, Zhu P, Wang B (2019) Molecular dynamics study of nonylphenol-substituted dodecyl sulfonate at air/water interface: role of steric effect of surfactant headgroups. J Mol Struct 1192:35–41
Ghoodarzi F, Zendehboudi S (2019) Effects of salt and surfactant on interfacial characteristics of water/oil systems: molecular dynamic simulations and dissipative particle dynamics. Ind Eng Chem Res 58:8817–8834
Shi P, Zhang H, Lin L, Song C, Chen Q, Li Z (2018) Molecular dynamics study of the effect of inorganic salts on the monolayer of four surfactants at the oil/water interface. J Dispers Sci Technol 39(12):1758–1766
Xu C, Wang H, Wang D, Zhu Y, Zhu X, Yu H (2021) Study on the mechanism of polyethylene oxide groups improving the foamability of anionic surfactants in hard water. Colloids Surf A Physicochem Eng Asp 613:126046
Nguyen NN, Nguyen AV, Dang LX (2017) The inhibition of methane hydrate formation by water alignment underneath surface adsorption of surfactants. Journal of Fuels 197:488–496
Sengupta S, Gera R, Egan C, Morzan UN, Versluis J, Hassanali A, Bakker HJ (2022) Observation of strong synergy in the interfacial water response of binary ionic and nonionic surfactant mixtures. J Phys Chem Lett 13(49):11391–11397
Parra JG, Iza P, Dominguez H, Schott E, Zarate X (2020) Effect of Triton X-100 surfactant on the interfacial activity of ionic surfactants SDS, CTAB and SDBS at the air/water interface: a study using molecular dynamic simulations. Colloids Surf A Physicochem Eng Asp 603:125284
Hantal G, Sega M, Horvai G, Jedlovszky P (2020) Role of the counterions in the surface tension of aqueous surfactant solutions. A Computer simulation study of alkali dodecyl sulfate systems. Adv Colloid Interface Sci 4:15
Cai HY, Zhang Y, Liu ZY, Li JG, Gong QT, Liao Q, Zhang L, Zhao S (2018) Molecular dynamics simulation of binary betaine and anionic surfactant mixtures at decane - water interface. J Mol Liq 266:82–89
Wu G, Zhu Q, Yuan C, Wang H, Li C, Sun S, Hu S (2017) Molecular dynamics simulation of the influence of polyacrylamide on the stability of sodium dodecyl sulfate foam. Chem Eng Sci 166:313–319
Parra JG, Aray YR, Iza P, Zarate X, Schott E (2019) Behavior of the SDS/1-butanol and SDS/2-butanol mixtures at the water/n-octane interface through molecular dynamics simulations. Chemical Physics 523:138–149
Poorsargol M, Alimohammadian M, Sohrabi B, Dehestani M (2019) Dispersion of graphene using surfactant mixtures: experimental and molecular dynamics simulation studies. Appl Surf Sci 464:440–450
Rowe RC, Sheskey PJ, Weller PJ (2006) Handbook of pharmaceutical excipients. Pharmaceutical Press, London
Wilhelm KP, Surber C, Maibach HI (1991) Effect of sodium lauryl sulfate-induced skin irritation on in vivo percutaneous penetration of four drugs. J Invest Dermatol 97:927–932
Taha MO, Nasser W, Ardakani A, AlKhatib HS (2008) Sodium lauryl sulfate impedes drug release from zinc-crosslinked alginate beads: switching from enteric coating release into biphasic profiles. Int J Pharm 350:291–300
Crison JR, Weiner ND, Amidon GL (1997) Dissolution media for in vitro testing of water-insoluble drugs: effect of surfactant purity and electrolyte on in vitro dissolution of carbamazepine in aqueous solutions of sodium lauryl sulfate. J Pharm Sci 86:384–388
Wang LH, Chowhan ZT (1990) Drug-excipient interactions resulting from powder mixing. V. Role of sodium lauryl sulfate. Int J Pharm 60:61–78
Alizadeh MN, Shayanfar A, Jouyban A (2018) Solubilization of drugs using sodium lauryl sulfate: experimental data and modeling. J Mol Liq 268:410–414
Dave RH, Patel HH, Donahue E, Patel AD (2013) To evaluate the change in release from solid dispersion using sodium lauryl sulfate and model drug sulfathiazole. Drug Dev Ind Pharm 39(10):1562–1572
Hicks E, Desgranges C, Delhommelle J (2014) Adsorption and diffusion of the antiparkinsonian drug amantadine in carbon nanotubes. Molecular Simulation 40:656–663
Gleed ML, Busath DD (2015) Why bound amantadine fails to inhibit proton conductance according to simulations of the drug-resistant influenza A M2 (S31N). J Phys Chem B 119:1225–1231
Xie S, Wang J, Yu X, Peng T, Yao K, Wang S, Liang D, Ke Y, Wang Z, Jiang H (2020) Site-directed mutations of anti-amantadine scFv antibody by molecular dynamics simulation: prediction and validation. J Mol Model 26:49
Konstantinidi A, Naziris N, Chountoulesi M, Kiriakidi S, Sartori B, Kolokouris D, Amentisch H, Mali G, Ntountaniotis D, Demetzos C, Mavromoustakos T, Kolocouris A (2018) Comparative perturbation effects exerted by the influenza M2 Protein Inhibitors Amantadine and the Spiro [pyrrolidine-2,2′-adamantane] variant AK13 to membrane bilayers studied using biophysical experiments and molecular dynamics simulations. J Phys Chem B 122(43):9877–9895
Habibzadeh Mashatooki M, Ghalami-Choobar B (2022) Improved delivery and competitive adsorption of paclitaxel and mitomycin C anticancer drugs on boron nitride nanoparticles: a molecular dynamics insight. Phys Chem Chem Phys 24(11):639–654
Habibzadeh Mashatooki M, Sardroodi JJ, Ebrahimzadeh AR (2019) The boron nitride nanotube, an ideal host structure for efficient immobilization and delivery of RNA aptamer: classical molecular dynamics simulation. J Inorg Organomet Polym Mater 30:789–800
Habibzadeh Mashatooki M, Sardroodi JJ, Ebrahimzadeh AR (2019) Molecular dynamics investigation of the interactions between RNA aptamer and graphene-monoxide/boron-nitride surfaces: applications to novel drug delivery systems. J Inorg Organomet Polym Mater 29:1252–1264
Habibzadeh Mashatooki M, Abbasi A, Sardroodi JJ (2020) In silico studies of the interaction between colon cancer receptor and RNA aptamer in complex with (1 0 1) facet of TiO2 nanosheet: a molecular dynamics study. Adsorption 26:941–954
Habibzadeh Mashatooki M, Ghalami-Choobar B (2021) Characterization of self-aggregated mitomycin C onto the boron-nitride nanotube as a drug delivery carrier: a molecular dynamics investigation. J Mol Liq 334:116065
Lee SH, Kim MJ, Ahn S, Koh B (2020) Purification of boron nitride nanotubes enhances biological application properties. Int J Mol Sci 21:1529
Fatemi SM, Foroutan M (2017) Review of recent studies on interactions between polymers and nanotubes using molecular dynamic simulation. J Iran Chem Soc 14:269–283
Guan M (2015) Gas uptake and thermal stability analysis of boron nitride and carbon nanotubes. Masters Abstracts International, p 47
Lahiri D, Rouzaud F, Richard T, Keshri AK, Bakshi SR, Kos L, Agarwal A (2010) Boron nitride nanotube reinforced polylactide–polycaprolactone copolymer composite: Mechanical properties and cytocompatibility with osteoblasts and macrophages in vitro. Acta Biomater 6(9):3524–3533
Ciofani G, Raffa V, Menciassi A, Cuschieri A (2008) Cytocompatibility, interactions, and uptake of polyethyleneimine-coated boron nitride nanotubes by living cells: confirmation of their potential for biomedical applications. Biotechnol Bioeng 101(4):850–858
Chen X, Wu P, Rousseas M, Okawa D, Gartner Z, Zettl A, Bertozzi CR (2009) Boron nitride nanotubes are noncytotoxic and can be functionalized for interaction with proteins and cells. J Am Chem Soc 131(3):890–891
Gao C, Feng P, Peng S, Shuai C (2017) Carbon nanotubes, graphene and boron nitride nanotubes reinforced bioactive ceramics for bone repair. Acta Biomater 61:1–20
Akram M, Osama M, La H, Salim M, Amiruddin Hashmi M, Din K (2023) Biophysical investigation of the interaction between NSAID ibuprofen and cationic biodegradable Cm-E2O2-Cm gemini surfactants. J Mol Liq 370:120972
Abdul Rub M, Azum N, Alfaifi SYM, Minhas M, Sakib H, Ackas Ali M, Mahbub S, Halim MA, Anamul Hoque M, Asiri AM (2023) Assessment of mixed micellization behavior of amitriptyline hydrochloride and TX-165 surfactant mixture in various media (aqueous/NaCl/urea). J Mol Liq 383:122073
Smida K, Albedah MA, Farooq Rashid R, Al-Qawasmi AR (2023) Molecular dynamics method for targeting α-synuclein aggregation induced Parkinson’s disease using boron nitride nanostructures. Eng Anal Bound Elem 146:89–95
Rharbi Y, Winnik MA (2002) Salt effects on solute exchange in sodium dodecyl sulfate micelles. J Am Chem Soc 124:2082
Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kalé L, Schulten K (2005) Scalable molecular dynamics with NAMD. J Comput Chem 26:1781–1802
Vanommeslaeghe K, Hatcher E, Acharya C, Kundu S, Zhong S, Shim J, Darian E, Mackerell AD (2009) CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J Comput Chem 31(4):671–690
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38
Zielesny A (2005) Chemistry Software Package ChemOffice Ultra 2005. J Chem Inf Model 45:1474–1477
Dupradeau FY, Pigache A, Zaffran T, Savineau C, Lelong R, Grivel N, Lelong D, Rosanski W, Cieplak P, The RED (2010) Tools: advances in RESP and ESP charge derivation and force field library building. Phys Chem Chem Phys 12:7821–7839
Zoete V, Cuendet MA, Grosdidier A, Michielin O (2011) Swissparam, a fast force field generation tool for small organic molecules. J Comput Chem 32(11):2359–2368
Dos Santos MAF, Habitzreuter MA, Schwade MH, Borrasca R, Antonacci M, Gonzatti GK, Netz PA, Barbosa MC (2019) Dynamical aspects of supercooled TIP3P-water in the grooves of DNA. J Chem Phys 150(23)
Zaragoza A, Gonzalez MA, Joly L, Lopez-Montero I, Canales MA, Benavides AL, Valeriani C (2019) Molecular dynamics study of nanoconfined TIP4P/2005 water: how confinement and temperature affect diffusion and viscosity. Physiol Chem Phys 21:13653–13667
Jorgensen WL, Madura JD (1983) Quantum and statistical mechanical studies of liquids. 25. Solvation and conformation of methanol in water. J Am Chem Soc 105:1407–1413
Lee H (2018) Structures, dynamics, and hydrogen-bond interactions of antifreeze proteins in TIP4P/Ice water and their dependence on force fields. PloS One 13(6):e0198887
Kubo R, Toda M, Hashitsume N (1991) Statistical Physics II: Nonequilibrium Statistical Mechanics2nd edn. Springer, New York
Feller SE, Zhang Y, Pastor RW, Brooks BR (1995) Constant pressure molecular dynamics simulation: the Langevin piston method. J Chem Phys 103:4613
McWilliams ADS, Martínez-Jiménez C, Shumard KR et al Dispersion and individualization of boron nitride nanotubes. J Mater Res 37:4459–4482
Lin S, Shih C-J, Sresht V, Govind Rajan A, Strano MS, Blankschtein D (2017) Understanding the colloidal dispersion stability of 1D and 2D materials: perspectives from molecular simulations and theoretical modeling. Adv Colloid Interface Sci 244:36–53
Jahanbakhsh Bonab P, Rastkar Ebrahimzadeh A, Jahanbin Sardroodi J (2021) Insights into the interactions and dynamics of a DES formed by phenyl propionic acid and choline chloride. Sci Rep 11:6384
Author contributions
F.S. and M.H.M. prepared the figures and tables. M.H.M. and B.G.C. wrote the main manuscript text. All authors reviewed the manuscript.
Funding
This work has been supported by the University of Guilan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This declaration is “not applicable.”
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shamizad, F., Habibzadeh Mashatooki, M. & Ghalami-Choobar, B. Biophysical assessment of amantadine and SDS surfactant mixture onto boron nitride nanotube: a molecular dynamics investigation. J Mol Model 29, 333 (2023). https://doi.org/10.1007/s00894-023-05736-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05736-9