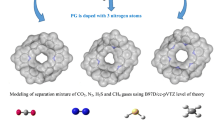
Abstract
Context
The adsorptive separation performances of fullerene pillared graphene nanocomposites (FPGNs) with tunable micro and meso porous morphology are investigated for the binary mixtures of CH4, H2, CO2 and N2 by using grand canonical Monte Carlo (GCMC) simulations. Different fullerene types are considered in designs as pillar to investigate the effects of porosity on the gas separation performances of FPGNs, and the GCMC simulations are performed for an equimolar binary mixture of CO2/H2, CO2/CH4, CO2/N2 and CH4/H2 inspired by industrial gas mixtures. It is found that CO2/N2, CO2/H2 and CH4/H2 selectivity of FPGNs are about 72, 410 and 145 at 298 K and 1 bar, which are higher than those for several adsorbent materials reported.
Methods
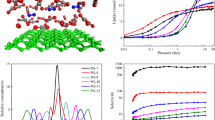
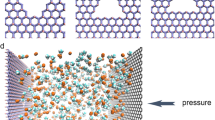
Five different FPGN models which contain covalently bonded periodical fullerene and graphene units were constructed using C60, C180, C320, C540 and C720 fullerenes, followed by geometry optimization using Open Babel. All GCMC simulations of adsorption were performed in the RASPA. The adsorption isotherms of FPGNs for pure gases are comparatively examined, and their performances are discussed based on the pore structure and isosteric heat of adsorption. Then, the separation factors of FPGNs for equimolar binary mixtures of these gases are elucidated from the difference in the heat of adsorption and the adsorption selectivity.
Graphical abstract








Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Chen Z, Kirlikovali KO, Idrees KB, Wasson MC, Farha OK (2022) Porous materials for hydrogen storage. Chemistry 8:693–716. https://doi.org/10.1016/J.CHEMPR.2022.01.012
Li Y, Wang Y, Fan W, Sun D (2022) Flexible metal-organic frameworks for gas storage and separation. Dalton Trans 51:4608–4618. https://doi.org/10.1039/D1DT03842G
Cao Z, Anjikar ND, Yang S (2022) Small-Pore Zeolite Membranes: A Review of Gas Separation Applications and Membrane Preparation. Sep 9:47. https://doi.org/10.3390/SEPARATIONS9020047.
Cui B, Fu G (2022) Process of metal-organic framework (MOF)/covalent-organic framework (COF) hybrids-based derivatives and their applications on energy transfer and storage. Nanoscale 14:1679–1699. https://doi.org/10.1039/D1NR07614K
Yürüm Y, Taralp A, Veziroglu TN (2009) Storage of hydrogen in nanostructured carbon materials. Int J Hydrogen Energy 34:3784–3798. https://doi.org/10.1016/j.ijhydene.2009.03.001
Lyu J, Kudiiarov V, Lider A (2020) An Overview of the Recent Progress in Modifications of Carbon Nanotubes for Hydrogen Adsorption. Nanomaterials 10. https://doi.org/10.3390/nano10020255
Majumdar S, Maurya M, Singh JK (2018) Adsorptive Separation of CO2 from Multicomponent Mixtures of Flue Gas in Carbon Nanotube Arrays: A Grand Canonical Monte Carlo Study. Energy Fuels 32:6090–6097. https://doi.org/10.1021/acs.energyfuels.8b00649
Barea E, Turra F, Navarro JAR (2011) Separation and Purification of Gases by MOFs. Met Fram Appl Catal Gas Storage 69–97. https://doi.org/10.1002/9783527635856.CH4
Das S, Feng J, Wang W (2020) Covalent Organic Frameworks in Separation. Annu Rev Chem Biomol Eng 11:131–153. https://doi.org/10.1146/ANNUREV-CHEMBIOENG-112019-084830
Kosinov N, Gascon J, Kapteijn F, Hensen EJM (2016) Recent developments in zeolite membranes for gas separation. J Memb Sci 499:65–79. https://doi.org/10.1016/j.memsci.2015.10.049
Huang L, Zhang M, Li C, Shi G (2015) Graphene-Based Membranes for Molecular Separation. J Phys Chem Lett 6:2806–2815. https://doi.org/10.1021/ACS.JPCLETT.5B00914
Gu C, Gao GH, Yu YX, Nitta T (2002) Simulation for separation of hydrogen and carbon monoxide by adsorption on single-walled carbon nanotubes. Fluid Phase Equilib 194–197:297–307. https://doi.org/10.1016/S0378-3812(01)00667-7
Sun P, Wang K, Zhu H (2016) Recent Developments in Graphene-Based Membranes: Structure, Mass-Transport Mechanism and Potential Applications. Adv Mater 28:2287–2310. https://doi.org/10.1002/ADMA.201502595
Gadipelli S, Guo ZX (2015) Graphene-based materials: Synthesis and gas sorption, storage and separation. Prog Mater Sci 69:1–60. https://doi.org/10.1016/j.pmatsci.2014.10.004
Attia NF, Jung M, Park J, Jang H, Lee K, Oh H (2020) Flexible nanoporous activated carbon cloth for achieving high H2, CH4, and CO2 storage capacities and selective CO2/CH4 separation. Chem Eng J 379. https://doi.org/10.1016/J.CEJ.2019.122367
Yang Y-B, Hao Q, Müller-Plathe F, Böhm MC (2020) Monte Carlo Simulations of SO2, H2S, and CO2 Adsorption in Charged Single-Walled Carbon Nanotube Arrays. J Phys Chem C 124:5838–5852. https://doi.org/10.1021/acs.jpcc.9b10424
Hong H, Guo Z, Yan D, Zhan H (2020) A Tröger’s base-derived microporous organic polymers containing pyrene units for selective adsorption of CO2 over N2 and CH4. Microporous Mesoporous Mater 294. https://doi.org/10.1016/J.MICROMESO.2019.109870
Krishna R (2012) Adsorptive separation of CO2/CH4/CO gas mixtures at high pressures. Microporous Mesoporous Mater 156:217–223. https://doi.org/10.1016/J.MICROMESO.2012.02.034
Maurya M, Singh JK (2017) A grand canonical Monte Carlo study of SO2 capture using functionalized bilayer graphene nanoribbons. J Chem Phys 146. https://doi.org/10.1063/1.4974309
Cheng H, Lei G, Dasgupta T, Punnathanam SN, Ayappa KG, Gu C, Gao GH, Yu YX, Nitta T, Lei G, Liu C, Li Q, Xu X, Lu X, Wang M, Luo G, Zhou S, Wang J, Xin H, Wang Z, Liu S, Wei S, Majumdar S, Maurya M, Singh JK, Wang Y, Wang W, Zhu S, Guo L, Zhang Z, Li P, Yang YB, Hao Q, Müller-Plathe F, Böhm MC (2018) Graphyne nanostructure as a potential adsorbent for separation of H2S/CH4 mixture: Combining grand canonical Monte Carlo simulations with ideal adsorbed solution theory. Chem Phys Lett 124:6090–6097. https://doi.org/10.1016/j.ces.2014.07.057
Feng C, Han JEW, Deng Y, Zhang B, Zhao X, Han D (2021) Key technology and application analysis of zeolite adsorption for energy storage and heat-mass transfer process: A review. Renew Sustain Energy Rev 144:110954. https://doi.org/10.1016/j.rser.2021.110954
Jiang D, Cooper VR, Dai S (2009) Porous Graphene as the Ultimate Membrane for Gas Separation. Nano Lett 9:4019–4024. https://doi.org/10.1021/NL9021946
Tao Y, Xue Q, Liu Z, Shan M, Ling C, Wu T, Li X (2014) Tunable Hydrogen Separation in Porous Graphene Membrane: First-Principle and Molecular Dynamic Simulation. ACS Appl Mater Interfaces 6:8048–8058. https://doi.org/10.1021/AM4058887
Bunch JS, Verbridge SS, Alden JS, van der Zande AM, Parpia JM, Craighead HG, McEuen PL (2008) Impermeable Atomic Membranes from Graphene Sheets. Nano Lett 8:2458–2462. https://doi.org/10.1021/NL801457B
Nieszporek K, Drach M (2014) Alkane separation using nanoporous graphene membranes. Phys Chem Chem Phys 17:1018–1024. https://doi.org/10.1039/C4CP02745K
Fischbein MD, Drndić M (2008) Electron beam nanosculpting of suspended graphene sheets. Appl Phys Lett 93:113107. https://doi.org/10.1063/1.2980518
Lehtinen O, Kotakoski J, Krasheninnikov AV, Tolvanen A, Nordlund K, Keinonen J (2010) Effects of ion bombardment on a two-dimensional target: Atomistic simulations of graphene irradiation. Phys Rev B 81:153401. https://doi.org/10.1103/PhysRevB.81.153401
Boutilier MSH, Sun C, O’Hern SC, Au H, Hadjiconstantinou NG, Karnik R (2014) Implications of Permeation through Intrinsic Defects in Graphene on the Design of Defect-Tolerant Membranes for Gas Separation. ACS Nano 8:841–849. https://doi.org/10.1021/NN405537U
Liu H, Chen Z, Dai S, Jiang DE (2015) Selectivity trend of gas separation through nanoporous graphene. J Solid State Chem 224:2–6. https://doi.org/10.1016/J.JSSC.2014.01.030
Sun C, Boutilier MSH, Au H, Poesio P, Bai B, Karnik R, Hadjiconstantinou NG (2014) Mechanisms of Molecular Permeation through Nanoporous Graphene Membranes. Langmuir 30:675–682. https://doi.org/10.1021/LA403969G
Paul R, Vincent M, Etacheri V, Roy AK (2019) Chapter 1 - Carbon nanotubes, graphene, porous carbon, and hybrid carbon-based materials: synthesis, properties, and functionalization for efficient energy storage. In: Paul R, Etacheri V, Wang Y, C.-T.B.T.-C.B.N. for A.T. and EES, Lin C (eds) Carbon Based Nanomater. Adv. Therm. Electrochem. Energy Storage Convers. Elsevier, pp 1–24. https://doi.org/10.1016/B978-0-12-814083-3.00001-9
Meena Devi J (2019) Simulation of graphene-fullerene nanohybrid structure. Bull Mater Sci 42:75. https://doi.org/10.1007/s12034-019-1753-0
Hassani A, Mosavian MT Hamed, Ahmadpour A, Farhadian N (2015) Hybrid molecular simulation of methane storage inside pillared graphene. J Chem Phys 142:234704. https://doi.org/10.1063/1.4922541
Ozturk Z, Baykasoglu C, Kirca M (2016) Sandwiched graphene-fullerene composite: A novel 3-D nanostructured material for hydrogen storage. Int J Hydrogen Energy 41:6403–6411. https://doi.org/10.1016/j.ijhydene.2016.03.042
Skarmoutsos I, Koukaras EN, Galiotis C, Froudakis GE, Klontzas E (2020) Porous carbon nanotube networks and pillared graphene materials exhibiting high SF6 adsorption uptake and separation selectivity of SF6/N2 fluid mixtures: A comparative molecular simulation study. Microporous Mesoporous Mater. 307:110464. https://doi.org/10.1016/J.MICROMESO.2020.110464
Etesami M, Nguyen MT, Yonezawa T, Tuantranont A, Somwangthanaroj A, Kheawhom S (2022) 3D carbon nanotubes-graphene hybrids for energy conversion and storage applications. Chem Eng J 446:137190. https://doi.org/10.1016/J.CEJ.2022.137190
Mert H, Deniz CU, Baykasoglu C (2020) Monte Carlo simulations of hydrogen adsorption in fullerene pillared graphene nanocomposites. Mol Simul 46:650–659. https://doi.org/10.1080/08927022.2020.1758696
Deniz CU, Mert H, Baykasoglu C (2021) Li-doped fullerene pillared graphene nanocomposites for enhancing hydrogen storage: A computational study. Comput Mater Sci 186:110023. https://doi.org/10.1016/j.commatsci.2020.110023
Baykasoglu C, Mert H, Deniz CU (2021) Grand canonical Monte Carlo simulations of methane adsorption in fullerene pillared graphene nanocomposites. J Mol Graph Model 106:107909. https://doi.org/10.1016/J.JMGM.2021.107909
Altintas C, Erucar I, Keskin S (2018) High-Throughput Computational Screening of the Metal Organic Framework Database for CH4/H2 Separations. ACS Appl Mater Interfaces 10:3668–3679. https://doi.org/10.1021/ACSAMI.7B18037
Yang Q, Li L, Tan W, Sun Y, Wang H, Ma J, Zhao X (2017) Exceptional high selectivity of hydrogen/methane separation on a phosphonate-based MOF membrane with exclusion of methane molecules. Chem Commun 53:9797–9800. https://doi.org/10.1039/C7CC05486F
Avci G, Velioglu S, Keskin S (2018) High-Throughput Screening of MOF Adsorbents and Membranes for H2 Purification and CO2 Capture. ACS Appl Mater Interfaces 10:33693–33706. https://doi.org/10.1021/ACSAMI.8B12746
Altintas C, Keskin S (2020) Role of partial charge assignment methods in high-throughput screening of MOF adsorbents and membranes for CO2/CH4 separation. Mol Syst Des Eng 5:532–543. https://doi.org/10.1039/C9ME00163H
Braun E, Zurhelle AF, Thijssen W, Schnell SK, Lin L-C, Kim J, Thompson JA, Smit B (2016) High-throughput computational screening of nanoporous adsorbents for CO2 capture from natural gas. Mol Syst Des Eng 1:175–188. https://doi.org/10.1039/C6ME00043F
Qiao Z, Peng C, Zhou J, Jiang J (2016) High-throughput computational screening of 137953 metal–organic frameworks for membrane separation of a CO2/N2/CH4 mixture. J Mater Chem A 4:15904–15912. https://doi.org/10.1039/C6TA06262H
Zhang H, Ren Z, Ye C, Dong Y (2018) An open-source code to generate carbon nanotube/graphene junctions. Comput Mater Sci 146:143–149. https://doi.org/10.1016/j.commatsci.2018.01.020
O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR (2011) Open Babel: An open chemical toolbox. J Cheminform 3:33. https://doi.org/10.1186/1758-2946-3-33
Dubbeldam D, Calero S, Vlugt TJH (2018) iRASPA: GPU-accelerated visualization software for materials scientists. Mol Simul 44:653–676. https://doi.org/10.1080/08927022.2018.1426855
Gelb LD, Gubbins KE (1999) Pore Size Distributions in Porous Glasses: A Computer Simulation Study. Langmuir 15:305–308. https://doi.org/10.1021/la9808418
Frenkel D, Smit B (2001) Understanding molecular simulation: from algorithms to applications. Academic Press, Elsevier Science. https://doi.org/10.1016/B978-0-12-267351-1.X5000-7
Dubbeldam D, Calero S, Ellis DE, Snurr RQ (2016) RASPA: Molecular simulation software for adsorption and diffusion in flexible nanoporous materials. Mol Simul 42:81–101. https://doi.org/10.1080/08927022.2015.1010082
Peng D-Y, Robinson DB (1976) A New Two-Constant Equation of State. Ind Eng Chem Fundam 15:59–64. https://doi.org/10.1021/i160057a011
Allen MP, Tildesley DJ (2017) Computer simulation of liquids: Second edition. Comput Simul Liq Second Ed 1–626. https://doi.org/10.1093/oso/9780198803195.001.0001
Steele WA (1974) The interaction of gases with solid surfaces. Pergamon Press, USA
Mahdizadeh SJ, Tayyari SF (2011) Influence of temperature, pressure, nanotube’s diameter and intertube distance on methane adsorption in homogeneous armchair open-ended SWCNT triangular arrays. Theor Chem Acc 128:231–240. https://doi.org/10.1007/s00214-010-0836-1
Potoff JJ, Siepmann JI (2001) Vapor-liquid equilibria of mixtures containing alkanes, carbon dioxide, and nitrogen. AIChE J 47:1676–1682. https://doi.org/10.1002/aic.690470719
Peng X, Zhou J, Wang W, Cao D (2010) Computer simulation for storage of methane and capture of carbon dioxide in carbon nanoscrolls by expansion of interlayer spacing. Carbon N Y 48:3760–3768. https://doi.org/10.1016/j.carbon.2010.06.038
Michels A, de Graaff W, Ten Seldam CA (1960) Virial coefficients of hydrogen and deuterium at temperatures between −175°C and +150°C. Conclusions from the second virial coefficient with regards to the intermolecular potential. Physica 26:393–408. https://doi.org/10.1016/0031-8914(60)90029-X
Ozturk Z, Baykasoglu C, Celebi AT, Kirca M, Mugan A, To AC (2015) Hydrogen storage in heat welded random CNT network structures. Int J Hydrogen Energy 40:403–411. https://doi.org/10.1016/j.ijhydene.2014.10.148
Da Wu C-D, Fang T-HH, Lo J-YY (2012) Effects of pressure, temperature, and geometric structure of pillared graphene on hydrogen storage capacity. Int J Hydrogen Energy 37:14211–14216. https://doi.org/10.1016/j.ijhydene.2012.07.040
Chung YG, Camp J, Haranczyk M, Sikora BJ, Bury W, Krungleviciute V, Yildirim T, Farha OK, Sholl DS, Snurr RQ (2014) Computation-ready, experimental metal-organic frameworks: A tool to enable high-throughput screening of nanoporous crystals. Chem Mater 26:6185–6192. https://doi.org/10.1021/cm502594j
Vlugt TJH, García-Pérez E, Dubbeldam D, Ban S, Calero S (2008) Computing the Heat of Adsorption using Molecular Simulations: The Effect of Strong Coulombic Interactions. J Chem Theory Comput 4:1107–1118. https://doi.org/10.1021/ct700342k
Mason JA, Sumida K, Herm ZR, Krishna R, Long JR (2011) Evaluating metal-organic frameworks for post-combustion carbon dioxide capture via temperature swing adsorption. Energy Environ Sci 4:3030–3040. https://doi.org/10.1039/c1ee01720a
Gotzias A, Kouvelos E, Sapalidis A (2018) Computing the temperature dependence of adsorption selectivity in porous solids. Surf Coatings Technol 350:95–100. https://doi.org/10.1016/J.SURFCOAT.2018.07.003
Myers AL (2002) Thermodynamics of adsorption in porous materials. AIChE J 48:145–160. https://doi.org/10.1002/aic.690480115
Gotzias A, Tylianakis E, Froudakis G, Steriotis T (2015) Adsorption in micro and mesoporous slit carbons with oxygen surface functionalities. Microporous Mesoporous Mater 209:141–149. https://doi.org/10.1016/j.micromeso.2014.08.052
Gotzias A (2016) The effect of gme topology on multicomponent adsorption in zeolitic imidazolate frameworks. Phys Chem Chem Phys 19:871–877. https://doi.org/10.1039/C6CP06036F
Kostoglou N, Koczwara C, Prehal C, Terziyska V, Babic B, Matovic B, Constantinides G, Tampaxis C, Charalambopoulou G, Steriotis T, Hinder S, Baker M, Polychronopoulou K, Doumanidis C, Paris O, Mitterer C, Rebholz C (2017) Nanoporous activated carbon cloth as a versatile material for hydrogen adsorption, selective gas separation and electrochemical energy storage. Nano Energy 40:49–64. https://doi.org/10.1016/J.NANOEN.2017.07.056
Arab P, Parrish E, Islamoʇlu T, El-Kaderi HM (2015) Synthesis and evaluation of porous azo-linked polymers for carbon dioxide capture and separation. J Mater Chem A 3:20586–20594. https://doi.org/10.1039/C5TA04308E
Wang J, Krishna R, Wu X, Sun Y, Deng S (2015) Polyfuran-Derived Microporous Carbons for Enhanced Adsorption of CO2 and CH4. Langmuir 31:9845–9852. https://doi.org/10.1021/ACS.LANGMUIR.5B02390/SUPPL_FILE/LA5B02390_SI_001.PDF
Yuan J, Wang Y, Tang M, Hao X, Liu J, Zhang G, Zhang Y (2023) Preparation of N, O co-doped carbon nanotubes and activated carbon composites with hierarchical porous structure for CO2 adsorption by coal pyrolysis. Fuel 333:126465. https://doi.org/10.1016/J.FUEL.2022.126465
Kostoglou N, Constantinides G, Charalambopoulou G, Steriotis T, Polychronopoulou K, Li Y, Liao K, Ryzhkov V, Mitterer C, Rebholz C (2015) Nanoporous spongy graphene: Potential applications for hydrogen adsorption and selective gas separation. Thin Solid Films 596:242–249. https://doi.org/10.1016/J.TSF.2015.06.060
Sircar S, Mohr R, Ristic C, Rao MB (1999) Isosteric Heat of Adsorption: Theory and Experiment. J Phys Chem B 103:6539–6546. https://doi.org/10.1021/JP9903817
Hsu SC, Lu C, Su F, Zeng W, Chen W (2010) Thermodynamics and regeneration studies of CO2 adsorption on multiwalled carbon nanotubes. Chem Eng Sci 65:1354–1361. https://doi.org/10.1016/j.ces.2009.10.005
Moormann R (1978) Gas-Solid Reactions. Von J. Szekely, J. W. Evans und H. Y. Sohn. Academic Press, New York 1976. 1. Aufl., XIII, 400 S., geb. $ 39.50, Angew. Chemie. 90:313–313. https://doi.org/10.1002/ANGE.19780900434
Stadie NP, Murialdo M, Ahn CC, Fultz B (2013) Anomalous isosteric enthalpy of adsorption of methane on zeolite-templated carbon. J Am Chem Soc 135:990–993. https://doi.org/10.1021/JA311415M/SUPPL_FILE/JA311415M_SI_001.PDF
Bhatia SK, Myers AL (2006) Optimum Conditions for Adsorptive Storage. Langmuir 22:1688–1700. https://doi.org/10.1021/LA0523816
Simmons JM, Wu H, Zhou W, Yildirim T (2011) Carbon capture in metal–organic frameworks—a comparative study. Energy Environ Sci 4:2177–2185. https://doi.org/10.1039/C0EE00700E
Yuan J, Liu X, Li X, Yu J (2021) Computer simulations for the adsorption and separation of CH4/H2/CO2/N2 gases by hybrid ultramicroporous materials. Mater Today Commun 26:101987. https://doi.org/10.1016/j.mtcomm.2020.101987
Yuan H, Chen J, Li D, Chen H, Chen Y (2019) 5 Ultramicropore-rich renewable porous carbon from biomass tar with excellent adsorption capacity and selectivity for CO2 capture. Chem Eng J 373:171–178. https://doi.org/10.1016/J.CEJ.2019.04.206
Jiang L, Wang P, Li M, Zhang P, Li J, Liu J, Ma Y, Ren H, Zhu G (2019) Construction of a Stable Crystalline Polyimide Porous Organic Framework for C2H2/C2H4 and CO2/N2 Separation. Chem. – A Eur J 25:9045–9051. https://doi.org/10.1002/CHEM.201900857
Garberoglio G, Pugno NM, Taioli S (2015) Gas adsorption and separation in realistic and idealized frameworks of organic pillared graphene: A comparative study. J Phys Chem C 119:1980–1987. https://doi.org/10.1021/JP511953P/SUPPL_FILE/JP511953P_SI_001.XYZ
Li XD, Hui Yang P, Huang XY, Liu XY, Yu JX, Chen Z (2022) Computational simulation study on adsorption and separation of CH4/H2 in five higher-valency covalent organic frameworks. Mater Today Commun 33:104374. https://doi.org/10.1016/J.MTCOMM.2022.104374
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
Humeyra Mert: Conceptualization, Methodology, Investigation, Visualization, Writing—Review & Editing
Celal Utku Deniz: Methodology, Software, Investigation, Visualization, Writing—Original Draft
Cengiz Baykasoğlu: Conceptualization, Methodology, Investigation, Writing—Review & Editing, Supervision.
Corresponding authors
Ethics declarations
Ethical approval
This declaration is “not applicable”.
Competing interests
The authors have no competing interests as defined by Springer, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mert, H., Deniz, C.U. & Baykasoglu, C. Adsorptive separation of CH4, H2, CO2, and N2 using fullerene pillared graphene nanocomposites: Insights from molecular simulations. J Mol Model 29, 315 (2023). https://doi.org/10.1007/s00894-023-05715-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05715-0