Abstract
Context
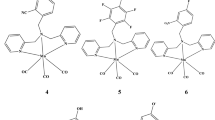
A large number of manganese and rhenium tricarbonyl complexes are known in literature along with various applications in different fields. CO-releasing molecules (CORMs) got recent research attention because CO can act as a prodrug for different diseases. CORMs offer the promising prospect of a safe and controllable amount of CO release. In this research work, we have explored the electronic properties of compounds such as bipyridine-related [Mn(CO)3] and [Re(CO)3] and we have compared the electronic properties of both manganese and rhenium tricarbonyl complexes in the light of carbon monoxide releasing tendency. The chosen Mn and Re metals have enough possibility to vary or play with ligands and design a new and novel CORM molecule. In this context, we have taken a range of 4,4′-disubstituted 2,2′ bipyridyl ligands (Rbpy, where R = NH2, tBu, OCH3, H, CF3, CN, NO2) to investigate CO’s liberation ability to identify and study such molecules. The calculated absorbance of designed complexes (1–14) shows visible/near-IR region (350–850 nm). The HOMO-LUMO energy gap of 7 (ΔE=2.40 eV) complex and for complex 14 (ΔE=2.28 eV) which is lesser in all complexes but the MLCT percentage is greater in Mn tricarbonyl complexes in comparison to Re tricarbonyl complexes. The calculated results of the FMO approach revealed that complex 7 and 14 have the lowest energy gap which is also in good agreement with DOSs and TDM results. The theoretically calculated results revealed that the both Mn and Re tricarbonyl complexes have a tendency for labialization of CO, but Mn tricarbonyl complexes are more prone to CO release because they have higher MLCT percentage.
Methods
In this research work, we have performed density functional theory (DFT) calculations to explore the physical properties of compounds such as bipyridine-related [Mn(CO)3] and [Re(CO)3] and we have compared the physical properties of both manganese and rhenium tricarbonyl complexes in the light of carbon monoxide releasing tendency. DFT-based calculations were performed by using B3LYP/LANL2DZ basis set followed by acetonitrile solvent using the conductor-like polarizable continuum model (CPCM) for different calculations. Various geometrical calculations were performed using the Gaussian16 suite of programs and the output results obtained from Gaussian16 were visualized using GaussView 5.0.16. The same level of theory was used for various calculations, including frontier molecular orbital (FMO) analysis, metal to ligand charge transfer (MLCT), density of state (DOS) calculations, and transition density of matrix (TDM) calculations. For specific calculations, GaussSum 2.2 software package was used to calculate the density of states, and the Multiwfn 3.8 program was used to analyze the transition density matrix, which is presented using heat maps for both electrons and holes.







Similar content being viewed by others
Data availability
We have used Gaussian 16, GaussSum and multiwfn 3.8 software.
References
Henke WC et al (2021) Synthesis, structural studies, and redox chemistry of bimetallic [Mn(CO)3] and [Re(CO)3] complexes. Dalton Trans 50(8):2746–2756
Blanco R, Ana M et al (2014) Photophysics of singlet and triplet intraligand excited states in [ReCl(CO)3(1-(2-pyridyl)-imidazo [1, 5-α] pyridine)] complexes. J Am Chem Soc 136(16):5963–5973
Wallace L, Rillema DP (1993) Photophysical properties of rhenium (I) tricarbonyl complexes containing alkyl-and aryl-substituted phenanthrolines as ligands. Inorg Chem 32(18):3836–3843
Léval A, Henrik J, Matthias B (2020) Manganese (i) κ 2-NN complex-catalyzed formic acid dehydrogenation. Catal Sci Technol 10(12):3931–3937
Shaver RJ et al (1995) Structure, physical and photophysical properties, and charge separation studies of [Re(bpm)(CO)3Ln+] complexes (L= CH3CN, py, MeQ+, py-PTZ). Inorg Chem 34(22):5446–5454
Schatzschneider U (2011) PhotoCORMs: light-triggered release of carbon monoxide from the coordination sphere of transition metal complexes for biological applications. InorganicaChimica Acta 374(1):19–23
Pinto MN et al (2017) Eradication of HT-29 colorectal adenocarcinoma cells by controlled photorelease of CO from a CO-releasing polymer (photoCORP-1) triggered by visible light through an optical fiber-based device. J Control Release 264:192–202
Alberto R et al (1998) A novel organometallic aqua complex of technetium for the labeling of biomolecules: synthesis of [99mTc(OH2)3(CO)3]+ from [99mTcO4]-in aqueous solution and its reaction with a bifunctional ligand. J Am Chem Soc 120(31):7987–7988
Hawecker J, Jean-Marie L, Raymond Z (1984) Electrocatalytic reduction of carbon dioxide mediated by Re(bipy)(CO)3Cl (bipy= 2, 2′-bipyridine). J Chem Soc Chem Commun 6:328–330
Bourrez M et al (2011) [Mn (bipyridyl)(CO)3Br]: an abundant metal carbonyl complex as efficient electrocatalyst for CO2 reduction. Angew Chem Int Ed 50(42):9903–9906
Sullivan BP et al (1985) One-and two-electron pathways in the electrocatalytic reduction of CO2 by fac-Re(bpy)(CO)3Cl (bpy= 2, 2′-bipyridine). J Chem Soc Chem Commun 20:1414–1416
Luong JC, Robert AF, Mark SW (1980) Ground-and excited-state oxidation-reduction chemistry of (triphenyltin)-and (triphenylgermanium) tricarbonyl (1, 10-phenanthroline) rhenium and related compounds. J Am Chem Soc 102(27):7892–7900
Caspar JV (1983) Thomas JM (1983) Application of the energy gap law to nonradiative, excited-state decay. J Phys Chem 87(6):952–957
Carriedo GA et al (1986) Synthesis of mono and binuclear carbonyl complexes of manganese with cyanide or thiocyanate ligands. X-ray crystal structure of [{fac-Mn(CO)3(phen)}2(μ-CN)] PF6. Inorganicachimica Acta 121(2):191–198
Takeda H et al (2008) Development of an efficient photocatalytic system for CO2 reduction using rhenium (I) complexes based on mechanistic studies. J Am Chem Soc 130(6):2023–2031
Smieja JM, Clifford PK (2010) Re (bipy-tBu)(CO) 3Cl− improved catalytic activity for reduction of carbon dioxide: IR-spectroelectrochemical and mechanistic studies. Inorg Chem 49(20):9283–9289
Gholamkhass B et al (2005) Architecture of supramolecular metal complexes for photocatalytic CO2 reduction: ruthenium− rhenium Bi-and tetranuclear complexes. Inorg Chem 44(7):2326–2336
Santoni M-P et al (2014) A light-harvesting polyoxometalate-polypyridine hybrid induces electron transfer as its Re(I) complex. Dalton Trans 43(19):6990–6993
Ettedgui J et al (2011) Photoreduction of carbon dioxide to carbon monoxide with hydrogen catalyzed by a rhenium (I) phenanthroline− polyoxometalate hybrid complex. J Am Chem Soc 133(2):188–190
Lee LC-C, Kam-Keung L, Kenneth K-WL (2017) Recent development of luminescent rhenium (I) tricarbonyl polypyridine complexes as cellular imaging reagents, anticancer drugs, and antibacterial agents. Dalton Trans 46(47):16357–16380
Smieja JM et al (2013) Manganese as a substitute for rhenium in CO2 reduction catalysts: the importance of acids. Inorg Chem 52(5):2484–2491
Fei H et al (2015) Photocatalytic CO2 reduction to formate using a Mn (I) molecular catalyst in a robust metal–organic framework. Inorg Chem 54(14):6821–6828
Ngo KT et al (2017) Turning on the protonation-first pathway for electrocatalytic CO2 reduction by manganese bipyridyl tricarbonyl complexes. J Am Chem Soc 139(7):2604–2618
Agarwal J et al (2015) Design of a catalytic active site for electrochemical CO2 reduction with Mn (I)-tricarbonyl species. Inorg Chem 54(11):5285–5294
Kadassery KJ, Samantha NM, David CL (2018) Bisphosphine phenol and phenolate complexes of Mn (I): manganese (I) catalyzed Tishchenko reaction. Dalton Trans 47(36):12652–12655
Kadassery KJ, David CL (2019) Pentacarbonylmethylmanganese (I) as a synthon for Mn (I) pincer catalysts. Dalton Trans 48(14):4467–4470
Li Y et al (2022) Head-to-head comparison of selected extra-and intracellular CO-releasing molecules on their CO-releasing and anti-inflammatory properties. ChemBioChem 23(1):e202100452
Romanski S et al (2012) Acyloxybutadiene tricarbonyl iron complexes as enzyme-triggered CO-releasing molecules (ET-CORMs): a structure–activity relationship study. Dalton Trans 41(45):13862–13875
Botov S et al (2013) Synthesis and performance of acyloxy-diene-Fe(CO)3 complexes with variable chain lengths as enzyme-triggered carbon monoxide-releasing molecules. Organometallics 32(13):3587–3594
Kunz PC et al (2013) Metal carbonyls supported on iron oxide nanoparticles to trigger the CO-gasotransmitter release by magnetic heating. Chem Commun 49(43):4896–4898
Rahul S, Amilan DJ (2021) New fluorinated manganese carbonyl complexes for light controlled carbon monoxide (CO) release and the use of benchtop 19F-NMR spectroscopy. InorganicaChimica Acta 516:120134
Rahul S et al (2019) Allosteric regulation in carbon monoxide (CO) release: anion responsive CO-releasing molecule (CORM) derived from (Terpyridine) phenol manganese tricarbonyl complex with colorimetric and fluorescence monitoring. Inorg Chem 58(16):10761–10768
Zobi F et al (2010) CO releasing properties and cytoprotective effect of cis-trans-[ReII(CO)2Br2L2] n complexes. Inorg Chem 49(16):7313–7322
Zobi F et al (2012) 17 e− rhenium dicarbonyl CO-releasing molecules on a cobalamin scaffold for biological application. Dalton Trans 41(2):370–378
Schindler K, Aurélien C, Fabio Z (2021) Aerobically stable and substitutionally labile a-diimine rhenium dicarbonyl complexes. RSC Adv 11(13):7511–7520
Aucott BJ et al (2017) Redox-tagged carbon monoxide-releasing molecules (CORMs): ferrocene-containing [Mn (C^ N)(CO) 4] complexes as a promising new CORM class. Inorg Chem 56(9):5431–5440
Motterlini R et al (2005) CORM-A1: a new pharmacologically active carbon monoxide-releasing molecule. FASEB J 19(2):1–24
Pitchumony TS et al (2010) Syntheses, structural characterization and CO releasing properties of boranocarbonate [H3BCO2H]− derivatives. Org Biomol Chem 8(21):4849–4854
Ahanger AA, Prawez SA, Tandan SK, Kumar D (2011) Naunyn-Schmiedeberg's Arch. Pharmacol 384:93–102
Motterlini R, Haas B, Foresti R (2012) Emerging concepts on the anti-inflammatory actions of carbon monoxide-releasing molecules (CO-RMs). Med Gas Res 2(1):1–12
Kottelat E, Zobi F (2017). Inorganics 5:24–43
Wright MA, Wright JA (2016). Dalton Trans 45:6801–6811
Indranil C, Samantha J, Carrington PKM (2014) Photodelivery of CO by designed photoCORMs: correlation between absorption in the visible region and metal–CO bond labilization in carbonyl complexes. Chem Med Chem 9(6):1266–1274
Carrington SJ et al (2016) A theranostic two-tone luminescent PhotoCORM derived from Re (I) and (2-pyridyl)-benzothiazole: trackable CO delivery to malignant cells. Inorg Chem 55(16):7852–7858
Carrington SJ, Indranil C, Pradip KM (2015) Exceptionally rapid CO release from a manganese (I) tricarbonyl complex derived from bis (4-chloro-phenylimino) acenaphthene upon exposure to visible light. Dalton Trans 44(31):13828–13834
Pierri AE et al (2012) A luminescent and biocompatible photoCORM. J Am Chem Soc 134(44):18197–18200
Marker SC et al (2018) Photoactivated in vitro anticancer activity of rhenium (I) tricarbonyl complexes bearing water-soluble phosphines. Inorg Chem 57(3):1311–1331
Takeda H, Osamu I (2010) Development of efficient photocatalytic systems for CO2 reduction using mononuclear and multinuclear metal complexes based on mechanistic studies. Coord Chem Rev 254(3-4):346–354
White JL et al (2015) Light-driven heterogeneous reduction of carbon dioxide: photocatalysts and photoelectrodes. Chem Rev 115(23):12888–12935
Thorp G, Flora L, Rebeca GM, Michael PC (2012) Organometallic complexes of transition metals in luminescent cell imaging applications. J Organomet Chem 714:12–21
Luengo A et al (2017) Trackable metallodrugs combining luminescent Re (I) and bioactive Au (I) fragments. Inorg Chem 56(24):15159–15170
Leonidova A, Gilles G (2014) Underestimated potential of organometallic rhenium complexes as anticancer agents. ACS Chem Biol 9(10):2180–2193
Kottelat E et al (2019) Correlation of MLCTs of Group 7 fac-[M (CO) 3]+ Complexes (M= Mn, Re) with Bipyridine, Pyridinylpyrazine, Azopyridine, and Pyridin-2-ylmethanimine type ligands for rational photoCORM design. Eur J Inorg Chem 33:3758–3768
Carrington SJ et al (2014) Synthesis and characterization of a “turn-on” photoCORM for trackable CO delivery to biological targets. ACS Med Chem Lett 5(12):1324–1328
Carrington SJ, Indranil C, Pradip KM (2013) Rapid CO release from a Mn (I) carbonyl complex derived from azopyridine upon exposure to visible light and its phototoxicity toward malignant cells. Chem Commun 49(96):11254–11256
Kurz P et al (2006) Ligand variations in [ReX (diimine)(CO)3] complexes: effects on photocatalytic CO2 reduction. Eur J Inorg Chem 2006(15):2966–2974
Henke WC et al (2020) Ultrafast spectroscopy of [Mn(CO)3] complexes: tuning the kinetics of light-driven CO release and solvent binding. Inorg Chem 59(4):2178–2187
Frisch ME, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone VPGA, Petersson GA, Nakatsuji HJRA, Li X (2016). Gaussian 16
Dennington RD, Keith TA, Millam JM (2008) GaussView 5.0, Gaussian. Inc, Wallingford 20
Kapturkiewicz A, Anna K, Olga G (2020) Heteroleptic Re(CO)2+ and Re(CO)3+ complexes with α-diimines: similarities and differences in their luminescence properties. RSC Adv 10(50):29642–29658
O’boyle NM (2008) Tenderholt AL Langner KM J. Comput Chem 29:839–845
Lu T, Feiwu C (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33(5):580–592
Ritu S et al (2022) Quest of new molecular frameworks for photoinduced carbon monoxide-releasing molecules: a computational prospective. Theor Chem Accounts 141(12):1–11
Rajneesh K et al (2023) Designing of gigantic first-order hyperpolarizability molecules via joining the promising organic fragments: a DFT study. J Mol Model 29(1):5
Cotton FA, Kraihanzel CS (1962) Vibrational spectra and bonding in metal carbonyls. I. Infrared spectra of phosphine-substituted group VI carbonyls in the CO stretching region. J Am Chem Soc 84(23):4432–4438
Santosh KY et al (2023) Designing excess electron compounds by substituting alkali metals to a small and versatile tetracyclic framework: a theoretical perspective. ACS Omega 8(8):7978–7988
Funding
Financial support has been given by Science and Engineering Research Board (SERB) (Ref. no. CRG/2019/001032) under core research grant for design of the study and collection, analysis, interpretation of data, and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
Ritu Seth performed the study and wrote the manuscript and Ajeet Singh executed the idea of the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Seth, R., Singh, A. Rational design of co-ordination compounds in combination of bipyridine type of ligands and group 7 metal (M = Mn, Re) for photoCORM: a DFT study. J Mol Model 29, 306 (2023). https://doi.org/10.1007/s00894-023-05712-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05712-3