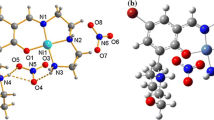
Abstract
Context
In contrast to un-catalyzed hydrolysis of organophosphorus (OP) compounds, metal ions or/and their complexes with chelating ligands show catalytic effects in several ways depending upon the nature of the metal, ligand, substrate, and medium. It is known that Cu(II)-en chelate containing copper complexes accelerate the hydrolysis of OP compounds. However, the mechanism for this rate enhancement in the Cu(II)-en chelate catalytic hydrolysis reaction of sarin remains unexplored. We have examined possible mechanisms involving a Cu(II)-en with hydroxide nucleophile for the reaction pathway of the hydrolysis of O-isopropyl methylphosphonofluoridate (sarin) computationally. The density functional (B3LYP) employed in this study has reproduced the experimental Gibb’s free energy of activation value 15.5 kcal/mol for alkaline hydrolysis of sarin. Earlier proposal of push–pull mechanism for metal ion chelate-catalyzed hydrolysis of OP compounds has been found to be unfavorable in the present study. The role of water molecules in catalyzing the hydrolysis of sarin with Cu(II)-en chelate is crucial. The catalytic process involving Cu(II)-en chelate with one water molecule is the more plausible pathway to achieve the hydrolysis of sarin with Cu(II)-en chelate complexes.
Methods
The most popular B3LYP method was used for optimization of given geometries. Except LANL2DZ for Cu atom, all the atoms are described using the 6–31 + G(d) basis set. The stability test has been performed for the wave functions as we are dealing with the open-shell molecules in order to ensure stable electronic configuration form, and the stable wavefunction is used as the initial configuration for the subsequent optimization. Harmonic frequency calculations and thermodynamic corrections were performed at the same level of theory. PCM method has been used for solvation effects. In order to ensure that each saddle point is linked to a minimum, IRC calculations were performed in forward and reverse directions to ensure the eigenvectors associated with the unique negative eigenvalues of the Hessian matrix. All energies discussed are solvated Gibbs free energies corrected to 298.15 K for the relative stability of the chemical structure. All calculations were performed using the Gaussian 09 code.







Similar content being viewed by others
References
Daniel KA, Kopff LA, Patterson EV (2008) Computational studies on the solvolysis of the chemical warfare agent VX. J Phys Org Chem 21:321–328
Yang Y-C, Szafraniec LL, Beaudry WT, Rohrbaugh DK (1990) Oxidative detoxification of phosphonothiolates. J Am Chem Soc 112:6621–6627
Simanenko YS, Savelova VA, Prop’eva TM, Mikhailov VA, Turovskaya MK, Karpichev EA, Popov AF, Gillitt ND, Bunton CA (2004) Bis(dialkylamide)hydrogen dibromobromate precursors of hypobromite ion in reactions with nerve and blister agent simulants. J Org Chem 69:9238–9240
Vorontsov AV, Davydov L, Reddy EP, Lion C, Savinov EN, Smirniotis PG (2002) Routes of photocatalytic destruction of chemical warfare agent simulants. New J Chem 26:732–744
Vorontsov AV, Chen Y-C, Smirniotis PG (2004) Photocatalytic oxidation of VX simulant 2-(butylamino) ethanethiol. J Hazard Mat B113:89–95
Michalkova A, Gorb L, IIchenko GM, Zhikol OA, Shishkin OV, Leszczynski J (2004) Adsorption of sarin and soman on dickite: an ab initio ONIOM study. J Phys Chem B 108:1918–1939
Keizer TS, Pue LJD, Parkin S, Atwood DA (2002) Catalytic dealkylation of phosphates with binuclear boron compounds. J Am Chem Soc 124:1864–1865
Hill CM, Li W-S, Thoden JB, Holden HM, Raushel FM (2003) Enhanced degradation of chemical warfare agents through molecular engineering of the phosphotriesterase active site. J Am Chem Soc 125:8990–8991
Amitai G, Adani R, Hershkovitz M, Bel P, Rabinovitz I, Meshulam H (2003) Degradation of VX and sulfur mustard by enzymatic haloperoxidation. J Appl Toxicol 23:225–233
Hoskins FCG, Walker JE, Dettbarn W-D, Wild JR (1995) Hydrolysis of tetriso by an enzyme derived from Pseudomonas diminuta as a model for the detoxication of O-ethyl S-(2-diisopropylaminoethyl) methylphosphonothiolate (VX). Biochem Pharmacol 49:711–715
Kiddle JJ, Mezyk SP (2004) Reductive destruction of chemical warfare agent simulants in water. J Phys Chem B 108:9568–9570
Aguila A, O’Shea KE, Tobien T, Asmus K-D (2001) Reactions of hydroxyl radical with dimethyl methylphosphonate and diethyl methylphosphonate. A Fundamental Mechanistic Study. J Phys Chem A 105:7834–7839
Šečkutė J, Menke JL, Emnett RJ, Patterson EV, Cramer CJ (2005) Ab initio molecular orbital, and density functional studies on the solvolysis of sarin and O, S-dimethyl methylphosphonothiolate, a VX-like compound. J Org Chem 70:8649–8660
Gustafson RL, Martell AE (1962) A kinetic study of the copper(II) chelate-catalyzed hydrolysis of isopropyl methylphosphonofluoridate (sarin). J Am Chem Soc 84:2309–2316
Larsson L (1957) The alkaline hydrolysis of isopropoxy-methyl-phosphoryl fluoride (sarin) and some analogues. Acta Chem Scand 12:1131–1142
Mazur A (1946) An enzyme in animal tissues capable of hydrolysing the phosphorus-fluorine bond of alkyl fluorophosphates. J Biol Chem 164:271–289
Courtney RC, Gustafson RL, Westerback SJ, Hyytiainen H Jr, Chaberek SC, Martell AE (1957) Metal chelate compounds as catalysts in the hydrolysis of isopropyl methylphosphonofluoridate and diisopropylphosphorofluoridate. J Am Chem Soc 79:3030–3036
Wagner-Jauregg T Jr, Hackley BE, Lies TA, Owens OO, Proper R (1955) Model reactions of phosphorus-containing enzyme inactivators. IV.1a The Catalytic Activity of Certain Metal Salts and Chelates in the Hydrolysis of Diisopropyl Fluorophosphate. J Am Chem Soc 77:922–929
Larsson L (1958) The catalytic effect of CrO42-, MoO42-, and WO42- on the hydrolysis of isopropoxy-methyl-phosphoryl fluoride (sarin). Acta Chem Scand 12:1226–1230
Epstein J, Rosenblatt DH (1958) Model reactions of phosphorus-containing enzyme inactivators. IV.1a The Catalytic Activity of Certain Metal Salts and Chelates in the Hydrolysis of Diisopropyl Fluorophosphate. J Am Chem Soc 80:3596–3598
Epstein J, Mosher WA (1968) Magnesium ion catalysis of hydrolysis of isopropyl methylphosphonofluoridate. Charge effect in metal ion catalysis. J Phys Chem 72:622–625
Hey RW, Govan N (1998) The [Cu(tmen) (OH) (OH2)]+ promoted hydrolysis of 2,4-dinitrophenyl diethyl phosphate and O-isopropyl methylphosphonofluoridate (Sarin) (tmen = N, N, N′, N′-tetramethyl-1,2-diaminoethane). Polyhedron 17:2079–2085
Swain CG, Brown JF (1952) Concerted displacement reactions. VII. The Mechanism of Acid—Base Catalysis in Non-aqueous Solvents. J Am Chem Soc 74:2534–2536
Healy PC, Kildea JD, Skelton BW, Waters AF, White AH (1991) -Lewis-base adducts of group 11 metal(I) compounds. 60. Binuclear adducts of copper(I) halides with 2-hindered pyridine bases. Acta Cryst C47:1721–1723
Becke AD (1993) Density-functional thermo chemistry III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Beck JM, Hadad CM (2008) Hydrolysis of nerve agents by model nucleophiles: a computational study. Chem Biol Interact 175:200–203
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98:11623–11627
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J Chem Phys 82:270–283
Wadt WR, Hay PJ (1985) Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J Chem Phys 82:284–298
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J Chem Phys 82:299–310
González C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94:5523–5527
González C, Schlegel HB (1991) Improved algorithms for reaction path following: higher-order implicit algorithms. J Chem Phys 95:5853–5860
Tomasi J, Persico M (1994) Molecular interactions in solution: an overview of methods based on continuous distributions of the solvent. Chem Rev 94:2027–2094
Cossi M, Barone V, Cammi R, Tomasi J (1996) Ab initio study of solvated molecules: a new implementation of the polarizable continuum model. Chem Phys Lett 255:327–335
Barone V, Cossi M, Tomasi J (1997) A new definition of cavities for the computation of solvation free energies by the polarizable continuum model. J Chem Phys 107:3210–3221
Barone V, Cossi M, Tomasi J (1998) Geometry optimization of molecular structures in solution by the polarizable continuum model. J Comput Chem 19:404–417
Cossi M, Barone V (1998) Analytical second derivatives of the free energy in solution by polarizable continuum models. J Chem Phys 109:6246–6254
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision E 01. Gaussian Inc, Wallingford CT
Acknowledgements
The authors thank DAE–BRNS, Mumbai, India, and CSIR (MSM, SIP), New Delhi, India, for the financial support of this work. One of the authors NBC is thankful to CSIR, New Delhi, India, for awarding senior research fellowship. We thank Mr. Rabindranath Lo for the valuable discussion.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception, design data collection, and analysis. The first draft of the manuscript was written by MASK, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khan, M.A.S., Bhanuchander, N., Reddy, J.S. et al. Computational exploration of Cu(II)-en chelate-catalyzed hydrolysis of O-isopropyl methylphosphonofluoridate. J Mol Model 29, 211 (2023). https://doi.org/10.1007/s00894-023-05614-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05614-4