Abstract
Context
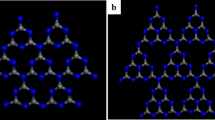
A steam-rich environment is a more promising application scenario for future coal-fired processes, while active sites are the key factor that determines the reactivity of carbonaceous fuels. The steam gasification process of carbon surfaces with different numbers of active sites (0, 12, 24, 36) was simulated using reactive molecular dynamics in the present study. The temperature for the decomposition of H2O and the gasification of carbon is determined using temperature-increasing simulation. The decomposition of H2O was influenced by two driving forces, thermodynamics and active sites on the carbon surface, which dominated the different reaction stages, leading to the observed segmentation phenomenon of the H2 production rate. The existence and number of initial active sites have a positive correlation with both two stages of the reaction, greatly reducing the activation energy. Residual OH groups play an important role in the gasification of carbon surfaces. The supply of OH groups through the cleavage of OH bonds in H2O is the rate-limiting step in the carbon gasification reaction. The adsorption preference at carbon defect sites was calculated using density functional theory. Two stable configurations (ether & semiquinone groups) can be formed with O atoms adsorbed on the carbon surface according to the number of active sites. This study will provide further insights into the tuning of active sites for advanced carbonaceous fuels or materials.
Methods
The large-scale atomic/molecule massively parallel simulator (LAMMPS) code combined with the reaction force-field method was used to carry out the ReaxFF molecular dynamics simulation, where the ReaxFF potentials were taken from Castro-Marcano, Weismiller and William. The initial configuration was built using Packmol, and the visualization of the calculation results was realized through Visual Molecular Dynamics (VMD). The timestep was set to 0.1 fs to detect the oxidation process with high precision. PWscf code in QUANTUM ESPRESSO (QE) package, was used to evaluate the relative stability of different possible intermediate configurations and the thermodynamic stability of gasification reactions. The projector augmented wave (PAW) and the generalized gradient approximation of Perdew-Burke-Ernzerhof (PBE-GGA) were adopted. Kinetic energy cutoffs of 50 Ry and 600 Ry, and a uniform mesh of 4 × 4 × 1 k-points were used.









Similar content being viewed by others
References
IEA (2021). Global Energy Review https://www.iea.org/reports/global-energy-review-2021/renewables
IEA, 2021World Energy Balances: Overview. https://www.iea.org/reports/world-energy-balances-overview.
Dueso C, Mayoral MC, Andrés JM, Escudero AI, Díez LI (2019) Towards oxy-steam combustion: The effect of increasing the steam concentration on coal reactivity. Fuel 239:534–546. https://doi.org/10.1016/j.fuel.2018.11.035
Yadav S, Mondal SS (2020) Modelling of oxy-pulverized coal combustion to access the influence of steam addition on combustion characteristics. Fuel 271:117611. https://doi.org/10.1016/j.fuel.2020.117611
Callide Oxyfuel Project. https://www.csenergy.com.au/what-we-do/generating-energy/callide-power-station/callide-oxyfuel-project.
Seepana S, Jayanti S (2010) Steam-moderated oxy-fuel combustion. Energ Conver Manage 51(10):1981–1988. https://doi.org/10.1016/j.enconman.2010.02.031
Mao Z, Zhang L, Zhu X, Pan C, Yi B, Zheng C (2016) Modeling of an oxy-coal flame under a steam-rich atmosphere. Appl Energy 161:112–123. https://doi.org/10.1016/j.apenergy.2015.10.018
Hossein Sahraei M, McCalden D, Hughes R, Ricardez-Sandoval LA (2014) A survey on current advanced IGCC power plant technologies, sensors and control systems. Fuel 137:245–259. https://doi.org/10.1016/j.fuel.2014.07.086
Cuenca MA, Anthony EJ (1995) Pressurized Fluidized Bed Combustion. Springer, Netherlands
Ahmed II, Gupta AK (2011) Kinetics of woodchips char gasification with steam and carbon dioxide. Appl Energy 88(5):1613–1619. https://doi.org/10.1016/j.apenergy.2010.11.007
Umemoto S, Kajitani S, Hara S (2013) Modeling of coal char gasification in coexistence of CO2 and H2O considering sharing of active sites. Fuel 103:14–21. https://doi.org/10.1016/j.fuel.2011.11.030
Liu H, Zhu H, Kaneko M, Kato S, Kojima T (2010) High-Temperature Gasification Reactivity with Steam of Coal Chars Derived under Various Pyrolysis Conditions in a Fluidized Bed. Energy Fuel 24(1):68–75. https://doi.org/10.1021/ef9004994
Qiao L, Xu J, Sane A, Gore J (2012) Multiphysics modeling of carbon gasification processes in a well-stirred reactor with detailed gas-phase chemistry. Combust Flame 159(4):1693–1707. https://doi.org/10.1016/j.combustflame.2011.12.002
Gil MV, Riaza J, Álvarez L, Pevida C, Pis JJ, Rubiera F (2012) A study of oxy-coal combustion with steam addition and biomass blending by thermogravimetric analysis. Journal of Thermal Analysis and Calorimetry 109(1):49–55. https://doi.org/10.1007/s10973-011-1342-y
Yi B, Zhang L, Huang F, Mao Z, Zheng C (2014) Effect of H2O on the combustion characteristics of pulverized coal in O2/CO2 atmosphere. Appl Energy 132:349–357. https://doi.org/10.1016/j.apenergy.2014.07.031
Roberts DG, Harris DJ (2007) Char gasification in mixtures of CO2 and H2O: Competition and inhibition. Fuel 86(17):2672–2678. https://doi.org/10.1016/j.fuel.2007.03.019
Goyal A, Zabransky RF, Rehmat A (1989) Gasification kinetics of Western Kentucky bituminous coal char. Industrial & Engineering Chemistry Research 28(12):1767–1778. https://doi.org/10.1021/ie00096a006
Bai Y, Gao Z, Chen N, Liu H, Yao J, Ma S, Shi X (2014) Elimination of small-sized Ag nanoparticles via rapid thermal annealing for high efficiency light trapping structure. Appl Surf Sci 315:1–7. https://doi.org/10.1016/j.apsusc.2014.07.029
Gao M, Yang Z, Wang Y, Bai Y, Li F, Xie K (2017) Impact of calcium on the synergistic effect for the reactivity of coal char gasification in H2O/CO2 mixtures. Fuel 189:312–321. https://doi.org/10.1016/j.fuel.2016.10.100
Tagutchou J-P, Van de Steene L, Escudero Sanz F, Salvador S (2013) Gasification of wood char in single and mixed atmospheres of H2O and CO2. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 35(13):1266–1276
Bai Y, Lv P, Yang X, Gao M, Zhu S, Yan L, Li F (2018) Gasification of coal char in H2O/CO2 atmospheres: Evolution of surface morphology and pore structure. Fuel 218:236–246. https://doi.org/10.1016/j.fuel.2017.11.105
Mengxia Q, Sheng S, Jian G, Zhijun S, Kai X, Jun X, Song H, Yi W, Jun X (2018) Effects of CO2/H2O on the characteristics of chars prepared in CO2/H2O/N2 atmospheres. Fuel Process Technol 173:262–269. https://doi.org/10.1016/j.fuproc.2018.01.003
Meconi GM, Zangi R (2020) Adsorption-induced clustering of CO2 on graphene. Phys Chem Chem Phys 22(37):21031–21041. https://doi.org/10.1039/D0CP03482G
Akilan R, Malarkodi M, Vijayakumar S, Gopalakrishnan S, Shankar R (2019) Modeling of 2-D hydrogen-edge capped defected & boron-doped defected graphene sheets for the adsorption of CO2, SO2 towards energy harvesting applications. Appl Surf Sci 463:596–609. https://doi.org/10.1016/j.apsusc.2018.08.179
Vallejos-Burgos F, Díaz-Pérez N, Silva-Villalobos Á, Jiménez R, García X, Radovic LR (2016) On the structural and reactivity differences between biomass- and coal-derived chars. Carbon 109:253–263. https://doi.org/10.1016/j.carbon.2016.08.012
Radovic LR, Bockrath B (2005) On the Chemical Nature of Graphene Edges: Origin of Stability and Potential for Magnetism in Carbon Materials. J Am Chem Soc 127(16):5917–5927. https://doi.org/10.1021/ja050124h
Radovic LR (2009) Active Sites in Graphene and the Mechanism of CO2 Formation in Carbon Oxidation. J Am Chem Soc 131(47):17166–17175. https://doi.org/10.1021/ja904731q
Shen A, Zou Y, Wang Q, Dryfe RAW, Huang X, Dou S, Dai L, Wang S (2014) Oxygen Reduction Reaction in a Droplet on Graphite: Direct Evidence that the Edge Is More Active than the Basal Plane. Angew Chem Int Ed 53(40):10804–10808. https://doi.org/10.1002/anie.201406695
Yamada Y, Kawai M, Yorimitsu H, Otsuka S, Takanashi M, Sato S (2018) Carbon Materials with Zigzag and Armchair Edges. ACS Appl Mater Interfaces 10(47):40710–40739. https://doi.org/10.1021/acsami.8b11022
Senda T, Yamada Y, Morimoto M, Nono N, Sogabe T, Kubo S, Sato S (2019) Analyses of oxidation process for isotropic pitch-based carbon fibers using model compounds. Carbon 142:311–326. https://doi.org/10.1016/j.carbon.2018.10.026
Hermann G, HÜttinger KJ (1986) Mechanism of water vapour gasification of carbon—A new model. Carbon 24(6):705–713. https://doi.org/10.1016/0008-6223(86)90178-8
Espinal JF, Mondragón F, Truong TN (2004) Density functional theory study of carbon-H2O reactions during gasification with steam, Prepr. Pap-Am Chem Soc, Div Fuel Chem 49(2):2
Kelemen SR, Freund H, Mims CA (1984) The dependence of H2O adsorption and reaction on the structure of the carbon substrate. Journal of Vacuum Science & Technology A 2(2):987–990. https://doi.org/10.1116/1.572498
Oyarzún AM, García-Carmona X, Radovic LR (2020) Kinetics of oxygen transfer reactions on the graphene surface. Part II. H2O vs. CO2. Carbon 164:85–99. https://doi.org/10.1016/j.carbon.2020.01.011
Montet GL, Myers GE (1968) Electron-microscopic investigation of the reaction of water vapor with single crystals of graphite—I. Reaction with edge atoms, Carbon 6(5):627–636. https://doi.org/10.1016/0008-6223(68)90006-7
Espinal JF, Mondragón F, Truong TN (2009) Thermodynamic evaluation of steam gasification mechanisms of carbonaceous materials. Carbon 47(13):3010–3018
Pittalis S, Proetto CR, Floris A, Sanna A, Bersier C, Burke K, Gross EKU (2011) Exact Conditions in Finite-Temperature Density-Functional Theory. Phys Rev Lett 107(16):163001. https://doi.org/10.1103/PhysRevLett.107.163001
Langreth DC, Perdew JP (1979) The gradient approximation to the exchange-correlation energy functional: A generalization that works. Solid State Commun 31(8):567–571. https://doi.org/10.1016/0038-1098(79)90254-0
Li K, Khanna R, Zhang H, Ma S, Liang Z, Li G, Barati M, Zhang J (2021) Thermal behaviour during initial stages of graphene oxidation: Implications for reaction kinetics and mechanisms. Chem Eng J 421:129742. https://doi.org/10.1016/j.cej.2021.129742
Li K, Khanna R, Zhang H, Conejo A, Ma S, Liang Z, Li G, Barati M, Zhang J (2021) Thermal behaviour, kinetics and mechanisms of CO2 interactions with graphene: An atomic scale reactive molecular dynamic study. Chem Eng J 425:131529. https://doi.org/10.1016/j.cej.2021.131529
Zeng Liang RK, Li K, Guo F, Ma Y, Zhang H, Yushan B, Bi Z, Zhang J (2022. (in press)) Impact of oxidants O2, steam and CO2 on graphene oxidation: A critical comparison of reaction kinetics and gasification behaviour. Chem Eng J 450:138045
Plimpton S (1995) Fast Parallel Algorithms for Short-Range Molecular Dynamics. J Comput Phys 1(117):1–19
Van Duin AC, Dasgupta S, Lorant F, Goddard WA (2001) ReaxFF: a reactive force field for hydrocarbons. Chem A Eur J 105(41):9396–9409
Castro-Marcano F, Kamat AM, Russo Jr MF, van Duin AC, Mathews JP, Combustion of an Illinois No. (2012) 6 coal char simulated using an atomistic char representation and the ReaxFF reactive force field. Combust Flame 159(3):1272–1285
Weismiller MR, van Duin AC, Lee J, Yetter RA (2010) ReaxFF reactive force field development and applications for molecular dynamics simulations of ammonia borane dehydrogenation and combustion. J Phys Chem A 114(17):5485–5492
An Q, Goddard III WA, Zybin SV, Jaramillo-Botero A, Zhou T (2013) Highly shocked polymer bonded explosives at a nonplanar interface: Hot-spot formation leading to detonation. J Phys Chem C 117(50):26551–26561
Chenoweth K, Van Duin AC, Goddard WA (2008) ReaxFF reactive force field for molecular dynamics simulations of hydrocarbon oxidation. Chem A Eur J 112(5):1040–1053
Bhoi S, Banerjee T, Mohanty K (2014) Molecular dynamic simulation of spontaneous combustion and pyrolysis of brown coal using ReaxFF. Fuel 136:326–333
Senftle TP, Hong S, Islam MM, Kylasa SB, Zheng Y, Shin YK, Junkermeier C, Engel-Herbert R, Janik MJ, Aktulga HM (2016) The ReaxFF reactive force-field: development, applications and future directions, npj Comput. Mater. 2(1):1–14
Zhang W, van Duin AC (2018) Improvement of the ReaxFF description for functionalized hydrocarbon/water weak interactions in the condensed phase. J Phys Chem B 122(14):4083–4092
Qiu Y, Zhong W, Shao Y, Yu A (2020) Reactive force field molecular dynamics (ReaxFF MD) simulation of coal oxy-fuel combustion. Powder Technol 361:337–348
Yang Z, Sun Y, Ma F, Lu Y, Zhao T (2020) Pyrolysis mechanisms of graphene oxide revealed by ReaxFF molecular dynamics simulation. Appl Surf Sci 509:145247
Liu J, Mao Q, Wang G, Xiao J, Zhong Q (2022) Removal and transformation mechanisms of nitrogen and sulfur in petcoke supercritical water gasification via ReaxFF simulation. Molecular Simulation 48(3):209–220. https://doi.org/10.1080/08927022.2021.2007908
Martínez L, Andrade R, Birgin EG, Martínez JM (2009) PACKMOL: A package for building initial configurations for molecular dynamics simulations. J Comput Chem 30(13):2157–2164. https://doi.org/10.1002/jcc.21224
Humphrey W, Dalke A, Schulten K (1996) VMD: Visual molecular dynamics. J Mol Graph 14(1):33–38. https://doi.org/10.1016/0263-7855(96)00018-5
Bal KM, Neyts EC (2016) Direct observation of realistic-temperature fuel combustion mechanisms in atomistic simulations. Chem Sci 7(8):5280–5286
Hong D, Si T, Li X, Guo X (2020) Reactive molecular dynamic simulations of the CO2 gasification effect on the oxy-fuel combustion of Zhundong coal char. Fuel Process Technol 199:106305. https://doi.org/10.1016/j.fuproc.2019.106305
Salmon E, Behar F, Lorant F, Hatcher PG, P.-M. (2009) Marquaire, Early maturation processes in coal. Part 1: Pyrolysis mass balance and structural evolution of coalified wood from the Morwell Brown Coal seam. Org Geochem 40(4):500–509. https://doi.org/10.1016/j.orggeochem.2009.01.004
E. Salmon, A.C.T. van Duin, F. Lorant, P.-M. Marquaire, W.A. Goddard, Early maturation processes in coal. Part 2: Reactive dynamics simulations using the ReaxFF reactive force field on Morwell Brown coal structures, Org Geochem 40(12) (2009) 1195-1209. https://doi.org/10.1016/j.orggeochem.2009.09.001.
Chang R, J.W. Thoman, Physical chemistry for the chemical sciences. University Science Books Canada2014
Scandolo S, Giannozzi P, Cavazzoni C, de Gironcoli S, Pasquarello A, Baroni S (2005) First-principles codes for computational crystallography in the Quantum-ESPRESSO package. Zeitschrift für Kristallographie-Crystalline Materials 220(5-6):574–579
Giannozzi P, Baroni S, Bonini N, Calandra M, Car R, Cavazzoni C, Ceresoli D, Chiarotti GL, Cococcioni M, Dabo I (2009) QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J Phys Condens Matter 21(39):395502
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77(18):3865
Liang Z, Li K, Wang Z, Bu Y, Zhang J (2021) Adsorption and reaction mechanisms of single and double H2O molecules on graphene surfaces with defects: a density functional theory study. Phys Chem Chem Phys 23(34):19071–19082. https://doi.org/10.1039/D1CP02595C
Irfan MF, Usman MR, Kusakabe K (2011) Coal gasification in CO2 atmosphere and its kinetics since 1948: A brief review. Energy 36(1):12–40. https://doi.org/10.1016/j.energy.2010.10.034
Heterogeneous Solid State Reactions. Chemical Kinetics of Solids1995, pp 137–164. https://doi.org/10.1002/9783527615537.ch06
Li K, Zhang J, Liu Z, Ning X, Wang T (2014) Gasification of Graphite and Coke in Carbon–Carbon Dioxide–Sodium or Potassium Carbonate Systems. Industrial & Engineering Chemistry Research 53(14):5737–5748. https://doi.org/10.1021/ie4039955
Gorbachev VM (1978) Remarks on the application of the combined Kolmogorov — Erofeev — Kazeev — Avrami — Mampel equation in the kinetics of non-isothermal transformations. Journal of Thermal Analysis and Calorimetry 13(3):509–514. https://doi.org/10.1007/bf01912390
Connors KA Chemical Kinetics. The Study of Reaction Rates in Solution VCH1990
Flanagan TB, Park CN, Oates WA (1995) Hysteresis in solid state reactions. Prog Solid State Chem 23(4):291–363. https://doi.org/10.1016/0079-6786(95)00006-G
Al Soubaihi RM, Saoud KM, Dutta J (2018) Critical Review of Low-Temperature CO Oxidation and Hysteresis Phenomenon on Heterogeneous Catalysts. Catalysts 8(12):660
Xuan W, Wang H, Yan S, Xia D (2022) Exploration on the steam gasification mechanism of waste PE plastics based on ReaxFF-MD and DFT methods. Fuel 315:123121. https://doi.org/10.1016/j.fuel.2021.123121
Chen J, Pan X, Li H, Jin H, Fan J (2020) Molecular dynamics investigation on the gasification of a coal particle in supercritical water. Int J Hydrogen Energy 45(7):4254–4267. https://doi.org/10.1016/j.ijhydene.2019.12.002
Yates Jr JT, McKee DW (1981) Kinetic isotope effect in the heterogeneous reaction of graphite with H2O (D2O). J Chem Phys 75(6):2711–2714. https://doi.org/10.1063/1.442339
Mims CA, Pabst JK (1987) Alkali-catalyzed carbon gasification kinetics: Unification of H2O, D2O, and CO2 reactivities. J Catal 107(1):209–220. https://doi.org/10.1016/0021-9517(87)90286-7
Barinov A, Malcioglu OB, Fabris S, Sun T, Gregoratti L, Dalmiglio M, Kiskinova M (2009) Initial stages of oxidation on graphitic surfaces: photoemission study and density functional theory calculations. J Phys Chem C 113(21):9009–9013
Sun T, Fabris S, Baroni S (2011) Surface precursors and reaction mechanisms for the thermal reduction of graphene basal surfaces oxidized by atomic oxygen. J Phys Chem C 115(11):4730–4737
Acknowledgements
This work was supported by the Young Elite Scientist Sponsorship Program by CAST (Grant numbers [YESS20210090]), the National Natural Science Foundation of China (Grant numbers [51974019]), Beijing Natural Science Foundation (Grant numbers [J210017]), China Baowu Low Carbon Metallurgy Innovation Foudation (Grant numbers [BWLCF202119]), and the National Key Research and Development Program of China (Grant numbers [2017YFB0304300] and [2017YFB0304303]). Computations were completed on the Niagara supercomputer at the SciNet HPC Consortium in the Compute/Calcul Canada national computing platform. SciNet is funded by the Canada Foundation for Innovation under the auspices of Compute Canada, the Government of Ontario, Ontario Research Fund - Research Excellence, and the University of Toronto. The authors acknowledge Prof. Mansoor Barati of the University of Toronto for the technical support.
Author information
Authors and Affiliations
Contributions
Zeng Liang: Data curation, Formal analysis, Writing original draft. Kejiang Li: Conceptualization, Discussion, Review & editing. Supervision. Feng Guo: Discussion. Hang Zhang: Software. Yushan Bu: Software, Visualization. Jianliang Zhang: Investigation, Supervision, Funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary informations
ESM 1:
Table S1. Fig. S1. Structural evolution of carbon surface cracking starting at 4450K. Fig. S2. Generation data for additional molecules (OH, H) as a function of time for (a) Pr; (b)12AS; (c) 24AS; (d) 36AS surfaces. Fig. S3. The (a) area; (b) perimeter; of the surface defect (light blue area) in Fig.6. Fig. S4. reaction intermediates of C-H2O system. Fig. S5. the energy difference between the 6AS-3O system with ether groups and the 6AS-3O system with semiquinone groups (Edifference = E6AS-semiquinone - E6AS-ether), which was eventually stabilized at 4.77 eV
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, Z., Li, K., Guo, F. et al. The Dynamic Nature of Graphene Active Sites in the H2O Gasification process: A ReaxFF and DFT Study. J Mol Model 29, 116 (2023). https://doi.org/10.1007/s00894-023-05527-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05527-2