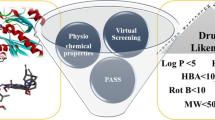
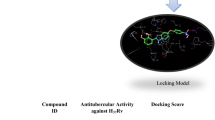
Abstract
Cases of drug-resistant tuberculosis (TB) have increased worldwide in the last few years, and it is a major threat to global TB control strategies and the human population. Mycobacterium tuberculosis is a common causative agent responsible for increasing cases of TB and as reported by WHO, approximately, 1.5 million death occurred from TB in 2020. Identification of new therapies against drug-resistant TB is an urgent need to be considered primarily. The current investigation aims to find the potential biogenic chalcone against the potential targets of drug-resistant TB via in silico approach. The ligand library of biogenic chalcones was screened against DprE1. Results of molecular docking and in silico ADMET prediction revealed that ZINC000005158606 has lead-like properties against the targeted protein. Pharmacophore modeling was done to identify the pharmacophoric features and their geometric distance present in ZINC000005158606. The binding stability study performed using molecular dynamics (MD) simulation of the DprE1-ZINC000005158606 complex revealed the conformational stability of the complex system over 100 ns with minimum deviation. Further, the in silico anti-TB sensitivity of ZINC000005158606 was found to be higher as compared to the standards against Mycobacterium tuberculosis. The overall in silico investigation indicated the potential of identified hit to act as a lead molecule against Mycobacterium tuberculosis.
Graphical Abstract








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and supplementary file.
References
Prasad SR, Satyanarayan ND, Shetty ASK, Thippeswamy B (2022) Synthesis, antimicrobial, and antitubercular evaluation of new Schiff bases with in silico ADMET and molecular docking studies. Eur J Chem 13:109–116. https://doi.org/10.5155/eurjchem.13.1.109-116.2216
Iacobino A, Fattorini L, Giannoni F (2020) Drug-resistant tuberculosis 2020: where we stand. Applied Sciences (Switzerland) 10
Kumar S, Sahu P, Jena L (2019) An in silico approach to identify potential inhibitors against multiple drug targets of Mycobacterium tuberculosis. Int J Mycobacteriol 8:252–261. https://doi.org/10.4103/ijmy.ijmy_109_19
Knechel NA (2009) Tuberculosis: Pathophysiology, clinical features, and diagnosis. Crit Care Nurse 29:34–43. https://doi.org/10.4037/ccn2009968
Sukhithasri V, Vinod V, Varma S, Biswas R (2014) Mycobacterium tuberculosis treatment modalities and recent insights. Curr Drug Deliv 11:744–752. https://doi.org/10.2174/1567201811666140619121728
Lee SH (2016) Tuberculosis infection and latent tuberculosis. Tuberc Respir Dis (Seoul) 79:201–206
Chikhale RV, Barmade MA, Murumkar PR, Yadav MR (2018) Overview of the development of DprE1 inhibitors for combating the menace of tuberculosis. J Med Chem 61:8563–8593
Fogel N (2015) Tuberculosis: a disease without boundaries. Tuberculosis 95:527–531
Kiazyk S, Ball TB (2017) Latent tuberculosis infection: an overview. Can Commun Dis Rep 43:. https://doi.org/10.1146/annurev
Niranjan Kumar, Srivastava R, Prakash A, Lynn AM (2021) Virtual screening and free energy estimation for identifying Mycobacterium tuberculosis flavoenzyme DprE1 inhibitors. J Mol Graph Model 102:. https://doi.org/10.1016/j.jmgm.2020.107770
Gao Y, Xie J, Tang R et al (2019) Identification of a pyrimidinetrione derivative as the potent DprE1 inhibitor by structure-based virtual ligand screening. Bioorg Chem 85:168–178. https://doi.org/10.1016/j.bioorg.2018.12.018
Liu R, Lyu X, Batt SM et al (2017) Determinants of the Inhibition of DprE1 and CYP2C9 by antitubercular thiophenes. Angewandte Chemie - International Edition 56:13011–13015. https://doi.org/10.1002/anie.201707324
Chhabra S, Kumar S, Parkesh R (2021) Chemical space exploration of DprE1 inhibitors using chemoinformatics and artificial intelligence. ACS Omega 6:14430–14441. https://doi.org/10.1021/acsomega.1c01314
Rudrapal M, Khan J, Dukhyil AA bin, et al (2021) Chalcone scaffolds, bioprecursors of flavonoids: chemistry, bioactivities, and pharmacokinetics. Molecules 26
Dan W, Dai J (2020) Recent developments of chalcones as potential antibacterial agents in medicinal chemistry. Eur J Med Chem 187
Yalcin G, Burmaoglu S, Yildiz I, Algul O (2018) Molecular docking studies on fluoro-substituted chalcones as potential DprE1 enzyme inhibitors. J Mol Struct 1164:50–56. https://doi.org/10.1016/j.molstruc.2018.02.087
Salehi B, Quispe C, Chamkhi I, et al (2021) Pharmacological properties of chalcones: a review of preclinical including molecular mechanisms and clinical evidence. Front Pharmacol 11
Rozmer Z, Perjési P (2016) Naturally occurring chalcones and their biological activities. Phytochem Rev 15:87–120
Kuber Banoth R, Thatikonda A (2020) A review on natural chalcones an update. Int J Pharm Sci Res 11:546. https://doi.org/10.13040/IJPSR.0975-8232.11(2).546-55
Al-Hazam H, Al-Shamkhan ZA, Al-Mayah AA, Hraishawi RM (2019) Microwave assisted synthesis characterization and study of some novel chalcones compounds derived from mefenamic acid. In: Journal of Physics: Conference Series. Institute of Physics Publishing
Anagani B, Singh J, Bassin JP, et al (2020) Identification and validation of the mode of action of the chalcone anti-mycobacterial compounds. Cell Surface 6:. https://doi.org/10.1016/j.tcsw.2020.100041
Lin Y-M, Zhou Y, Flavin MT et al (2002) Chalcones and flavonoids as anti-tuberculosis agents. Bioorg Med Chem 10:2795–2802
Ventura TLB, Calixto SD, de Azevedo A-V et al (2015) Antimycobacterial and anti-inflammatory activities of substituted chalcones focusing on an anti-tuberculosis dual treatment approach. Molecules 20:8072–8093. https://doi.org/10.3390/molecules20058072
Maharaj Y, Bhakat S, Soliman M (2014) Computer-aided identification of novel DprE1 inhibitors as potential anti-TB lead compounds: a hybrid virtual-screening and molecular dynamics approach. Lett Drug Des Discov 12:302–313. https://doi.org/10.2174/1570180811666141001005536
Batt SM, Jabeen T, Bhowruth V et al (2012) Structural basis of inhibition of Mycobacterium tuberculosis DprE1 by benzothiazinone inhibitors. Proc Natl Acad Sci U S A 109:11354–11359. https://doi.org/10.1073/PNAS.1205735109/-/DCSUPPLEMENTAL
Sharma M, Vyas VK, Bhatt S, Ghate MD (2022) Therapeutic potential of 4-substituted coumarins: a conspectus. Eur J Med Chem Rep 6:100086. https://doi.org/10.1016/J.EJMCR.2022.100086
Basheera S, Sivanandan S, Kamalan BC (2021) Anti-tuberculosis activity in Punica granatum in silico validation and identification of lead molecules. Indian J Pharm Sci 83:316–330. https://doi.org/10.36468/PHARMACEUTICAL-SCIENCES.778
Hebbar NU, Patil AR, Gudimani P et al (2022) Click approach for synthesis of 3,4-dihydro-2(1H) quinolinone, coumarin moored 1,2,3-triazoles as inhibitor of mycobacteria tuberculosis H37RV, their antioxidant, cytotoxicity and in-silico studies. J Mol Struct 1269:133795. https://doi.org/10.1016/J.MOLSTRUC.2022.133795
Dhameliya TM, Bhakhar KA, Gajjar ND, et al (2022) Recent advancements and developments in search of anti-tuberculosis agents: a quinquennial update and future directions. J Mol Struct 1248
Shareef THMA, Masood MD (2022) Phytochemical and molecular docking studies on indigenous herbs Glycyrrhiza glabra, Terminalia chebula and Hamdard joshanda. Int J Pharm Investig 12:07–14. https://doi.org/10.5530/ijpi.2022.1.2
Ramesh D, Joji A, Vijayakumar BG et al (2020) Indole chalcones: design, synthesis, in vitro and in silico evaluation against Mycobacterium tuberculosis. Eur J Med Chem 198:112358. https://doi.org/10.1016/J.EJMECH.2020.112358
Dassault Systèmes (2020) BIOVIA Discovery Studio Visualizr
Hashemzadeh P, Ghorbanzadeh V, Lashgarian HE et al (2020) Harnessing bioinformatic approaches to design novel multi-epitope subunit vaccine against Leishmania infantum. Int J Pept Res Ther 26:1417–1428. https://doi.org/10.1007/s10989-019-09949-6
Dym O, Eisenberg D, Yeates TO (2006) Detection of errors in protein models. Int Tables for Crystallography 520–530. https://doi.org/10.1107/97809553602060000709
Bhowmik D, Nandi R, Prakash A, Kumar D (2021) Evaluation of flavonoids as 2019-nCoV cell entry inhibitor through molecular docking and pharmacological analysis. Heliyon 7:. https://doi.org/10.1016/J.HELIYON.2021.E06515
Wiederstein M, Sippl MJ (2007) ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res 35:. https://doi.org/10.1093/nar/gkm290
Roman Laskowski BA, Macarthur MW, Thornton JM (1983) Computer Programs PROCHECK: a program to check the stereochemicai quality of protein structures. J Appl Crystallogr 26:283–291
Irwin JJ, Shoichet BK (2005) ZINC-a free database of commercially available compounds for virtual screening. J Chem Inf Model 45:177–182. https://doi.org/10.1021/ci049714
Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules OPEN. Sci Rep 7:42717. https://doi.org/10.1038/srep42717
Magnet S, Hartkoorn RC, Székely R et al (2010) Leads for antitubercular compounds from kinase inhibitor library screens. Tuberculosis 90:354–360. https://doi.org/10.1016/j.tube.2010.09.001
Eberhardt J, Santos-Martins D, Tillack AF, Forli S (2021) AutoDock Vina 1.2.0: new docking methods, expanded force field, and python bindings. J Chem Inf Model 61:3891–3898. https://doi.org/10.1021/acs.jcim.1c00203
Ikwu FA, Isyaku Y, Obadawo BS et al (2020) In silico design and molecular docking study of CDK2 inhibitors with potent cytotoxic activity against HCT116 colorectal cancer cell line. J Gen Eng Biotechnol 18:1–12. https://doi.org/10.1186/s43141-020-00066-2
Muniz LS, da Silva S, Pita R (2022) In silico studies revealed interaction mechanisms of benzylidene–acrylohydrazide derivatives and nsP2 CHIKV. New J Chem 46:6414–6423. https://doi.org/10.1016/j.ejmech.2018.02.054
O’boyle NM, Banck M, James CA et al (2011) Open Babel an open chemical toolbox. J Cheminform 3:1–14. https://doi.org/10.1186/1758-2946-3-33
Forli S, Huey R, Pique ME et al (2016) Computational protein-ligand docking and virtual drug screening with the AutoDock suite. Nat Protoc 11:905–919. https://doi.org/10.1038/nprot.2016.051
van de Waterbeemd H, Gifford E (2003) ADMET in silico modelling: towards prediction paradise? Nat Rev Drug Discov 2003 2:3 2:192–204. https://doi.org/10.1038/nrd1032
The role of ADME & toxicology studies in drug discovery & development - The Connected Lab. https://www.thermofisher.com/blog/connectedlab/the-role-of-adme-toxicology-studies-in-drug-discovery-development/. Accessed 10 Aug 2022
Shivanika C, Deepak Kumar S, Ragunathan V et al (2022) Molecular docking, validation, dynamics simulations, and pharmacokinetic prediction of natural compounds against the SARS-CoV-2 main-protease. J Biomol Struct Dyn 40:585–611. https://doi.org/10.1080/07391102.2020.1815584
Abdusalam AAA, Murugaiyah V (2020) Identification of potential inhibitors of 3CL protease of SARS-CoV-2 from ZINC database by molecular docking-based virtual screening. Front Mol Biosci 7:. https://doi.org/10.3389/fmolb.2020.603037
Azam F (2021) Elucidation of teicoplanin interactions with drug targets related to COVID-19. Antibiotics 10:. https://doi.org/10.3390/antibiotics10070856
Alici H, Tahtaci H, Demir K (2022) Design and various in silico studies of the novel curcumin derivatives as potential candidates against COVID-19 -associated main enzymes. Comput Biol Chem 98:107657. https://doi.org/10.1016/j.compbiolchem.2022.107657
Bagal VK, Rathod SS, Mulla MM, et al (2023) Exploration of bioactive molecules from Tinospora cordifolia and Actinidia deliciosa as an immunity modulator via molecular docking and molecular dynamics simulation study. Nat Prod Res 1–5. https://doi.org/10.1080/14786419.2023.2165076
Ramírez D, Caballero J (2018) Is it reliable to take the molecular docking top scoring position as the best solution without considering available structural data? Molecules 23:. https://doi.org/10.3390/molecules23051038
Thilagavathi R, Mancera RL (2010) Ligand-protein cross-docking with water molecules. J Chem Inf Model 50:415–421. https://doi.org/10.1021/ci900345h
Shen C, Hu X, Gao J, et al (2021) The impact of cross-docked poses on performance of machine learning classifier for protein–ligand binding pose prediction. J Cheminform 13:. https://doi.org/10.1186/s13321-021-00560-w
OSF | Brown Lab, Public Wiki. https://osf.io/82n73/wiki/Docking%2C%20Re-docking%2C%20and%20Cross%20Docking/. Accessed 29 Jan 2023
Wierbowski SD, Wingert BM, Zheng J, Camacho CJ (2020) Cross-docking benchmark for automated pose and ranking prediction of ligand binding. Protein Sci 29:298–305. https://doi.org/10.1002/pro.3784
Wan H (2013) What ADME tests should be conducted for preclinical studies? ADMET DMPK 1:19–28. https://doi.org/10.5599/ADMET.1.3.9
Pires DEV, Blundell TL, Ascher DB, Iga UK (2015) pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. in silico and molecular docking studies of black pepper phyto-constituents against. EmrD efflux pump of E coli 58:4066–4072. https://doi.org/10.1021/acs.jmedchem.5b00104
Matias M, Fortuna A, Bicker J et al (2017) Screening of pharmacokinetic properties of fifty dihydropyrimidin(thi)one derivatives using a combo of in vitro and in silico assays. Eur J Pharm Sci 109:334–346. https://doi.org/10.1016/j.ejps.2017.08.023
Katiyar K, Srivastava RK, Nath R (2021) Identification of novel anti-cryptosporidial inhibitors through a combined approach of pharmacophore modeling, virtual screening, and molecular docking. Inform Med Unlocked 24:. https://doi.org/10.1016/j.imu.2021.100583
Schneidman-Duhovny D, Dror O, Inbar Y, et al (2008) PharmaGist: a web server for ligand-based pharmacophore detection. Nucleic Acids Res 36:. https://doi.org/10.1093/nar/gkn187
Dror O, Schneidman-Duhovny D, Inbar Y et al (2009) Novel approach for efficient pharmacophore-based virtual screening: method and applications. J Chem Inf Model 49:2333–2343. https://doi.org/10.1021/ci900263d
Chen YC (2015) Beware of docking! Trends Pharmacol Sci 36:78–95
Schneidman-Duhovny D, Dror O, Inbar Y, et al (2008) Deterministic pharmacophore detection via multiple flexible alignment of drug-like molecules. In: Journal of Computational Biology. 737–754
Joshi M, Singh S, Patel S et al (2018) Identification of small molecule activators for ErbB 4 receptor to enhance oligodendrocyte regeneration by in silico approach. Comput Toxicol 8:13–20. https://doi.org/10.1016/j.comtox.2018.08.004
dos Santos IVF, Borges RS, Silva GM, et al (2022) Hierarchical virtual screening based on rocaglamide derivatives to discover new potential anti-skin cancer agents. Front Mol Biosci 9:. https://doi.org/10.3389/fmolb.2022.836572
Koes DR, Camacho CJ (2012) ZINCPharmer: Pharmacophore search of the ZINC database. Nucleic Acids Res 40:. https://doi.org/10.1093/nar/gks378
Pol-Fachin L, Fernandes CL, Verli H (2009) GROMOS96 43a1 performance on the characterization of glycoprotein conformational ensembles through molecular dynamics simulations. Carbohydr Res 344:491–500. https://doi.org/10.1016/j.carres.2008.12.025
WebGro | UAMS. https://simlab.uams.edu/. Accessed 22 May 2022
Kalimuthu AK, Panneerselvam T, Pavadai P, et al (2021) Pharmacoinformatics-based investigation of bioactive compounds of Rasam (South Indian recipe) against human cancer. Sci Rep 11:. https://doi.org/10.1038/s41598-021-01008-9
Tumskiy RS, Tumskaia AV (2021) Multistep rational molecular design and combined docking for discovery of novel classes of inhibitors of SARS-CoV-2 main protease 3CLpro. Chem Phys Lett 780:138894. https://doi.org/10.1016/j.cplett.2021.138894
Vishvakarma VK, Pal S, Singh P, Bahadur I (2022) Interactions between main protease of SARS-CoV-2 and testosterone or progesterone using computational approach. J Mol Struct 1251:. https://doi.org/10.1016/j.molstruc.2021.131965
Schüttelkopf AW, van Aalten DMF (2004) PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr D Biol Crystallogr 60:1355–1363. https://doi.org/10.1107/S0907444904011679
Raman APS, Kumari K, Jain P et al (2022) In silico evaluation of binding of 2-deoxy-D-glucose with Mpro of nCoV to combat COVID-19. Pharmaceutics 14:135. https://doi.org/10.3390/pharmaceutics14010135
Izadi S, Anandakrishnan R, Onufriev AV (2014) Building water models: a different approach. J Phys Chem Lett 5:3863–3871. https://doi.org/10.1021/jz501780a
Rathod S, Shinde K, Porlekar J et al (2023) Computational exploration of anti-cancer potential of flavonoids against cyclin-dependent kinase 8: an in silico molecular docking and dynamic approach. ACS Omega 8:391–409. https://doi.org/10.1021/acsomega.2c04837
Gorai S, Junghare V, Kundu K et al (2022) Synthesis of dihydrobenzofuro[3,2-b]chromenes as potential 3CL pro Inhibitors of SARS-CoV-2: a molecular docking and molecular dynamics study. ChemMedChem 17:e202100782. https://doi.org/10.1002/cmdc.202100782
NosÉ S (2002) A molecular dynamics method for simulations in the canonical ensemble. Mol Phys 100:191–198. https://doi.org/10.1080/00268970110089108
Huang C, Li C, Choi PYK et al (2011) A novel method for molecular dynamics simulation in the isothermal-isobaric ensemble. Mol Phys 109:191–202. https://doi.org/10.1080/00268976.2010.513345
Bepari AK, Reza HM (2021) Identification of a novel inhibitor of SARS-CoV-2 3CL-PRO through virtual screening and molecular dynamics simulation. PeerJ 9:e11261. https://doi.org/10.7717/peerj.11261
Berendsen HJC, Postma JPM, van Gunsteren WF et al (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81:3684–3690. https://doi.org/10.1063/1.448118
Parrinello M, Rahman A (1981) Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys 52:7182–7190. https://doi.org/10.1063/1.328693
Kushwaha PP, Singh AK, Bansal T et al (2021) Identification of natural inhibitors against SARS-CoV-2 drugable targets using molecular docking, Molecular Dynamics Simulation, and MM-PBSA Approach. Front Cell Infect Microbiol 11:730288. https://doi.org/10.3389/fcimb.2021.730288
Jiang Z, You L, Dou W et al (2019) Effects of an electric field on the conformational transition of the protein: a molecular dynamics simulation study. Polymers (Basel) 11:282. https://doi.org/10.3390/polym11020282
Afgan E, Baker D, Batut B et al (2018) The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res 46:W537–W544. https://doi.org/10.1093/nar/gky379
Grant BJ, Rodrigues APC, ElSawy KM et al (2006) Bio3d: an R package for the comparative analysis of protein structures. Bioinformatics 22:2695–2696. https://doi.org/10.1093/bioinformatics/btl461
Wang RR, Ma Y, Du S et al (2019) Exploring the reason for increased activity of SHP2 caused by D61Y mutation through molecular dynamics. Comput Biol Chem 78:133–143. https://doi.org/10.1016/j.compbiolchem.2018.10.013
Kumar N, Awasthi A, Kumari A et al (2022) Antitussive noscapine and antiviral drug conjugates as arsenal against COVID-19: a comprehensive chemoinformatics analysis. J Biomol Struct Dyn 40:101–116. https://doi.org/10.1080/07391102.2020.1808072
Wang Z, Wang X, Li Y et al (2019) farPPI: a webserver for accurate prediction of protein-ligand binding structures for small-molecule PPI inhibitors by MM/PB(GB)SA methods. Bioinformatics 35:1777–1779. https://doi.org/10.1093/BIOINFORMATICS/BTY879
Grassoa G, di Gregorio A, Mavkov B, et al (2021) Fragmented blind docking: a novel protein–ligand binding prediction protocol. J Biomol Struct Dyn 1–10. https://doi.org/10.1080/07391102.2021.1988709/FORMAT/EPUB
Yalçın S, Yalçınkaya S, Ercan F (2021) In silico detection of inhibitor potential of Passiflora compounds against SARS-Cov-2(Covid-19) main protease by using molecular docking and dynamic analyses. J Mol Struct 1240:. https://doi.org/10.1016/j.molstruc.2021.130556
Bansod S, Raj N, R A, et al (2022) Molecular docking and molecular dynamics simulation identify a novel radicicol derivative that predicts exclusive binding to Plasmodium falciparum Topoisomerase VIB. J Biomol Struct Dyn 40:6939–6951https://doi.org/10.1080/07391102.2021.1891970
Venkateshan M, Muthu M, Suresh J, Ranjith Kumar R (2020) Azafluorene derivatives as inhibitors of SARS CoV-2 RdRp synthesis, physicochemical, quantum chemical, modeling and molecular docking analysis. J Mol Struct 1220:128741. https://doi.org/10.1016/j.molstruc.2020.128741
Mohapatra RK, Azam M, Mohapatra PK et al (2022) Computational studies on potential new anti-Covid-19 agents with a multi-target mode of action. J King Saud Univ Sci 34:102086. https://doi.org/10.1016/J.JKSUS.2022.102086
Pires DEV, Ascher DB (2020) MycoCSM: using graph-based signatures to identify safe potent hits against Mycobacteria. J Chem Inf Model 60:3450–3456. https://doi.org/10.1021/acs.jcim.0c00362
Rajasekhar S, Karuppasamy R, Chanda K (2021) Exploration of potential inhibitors for tuberculosis via structure-based drug design, molecular docking, and molecular dynamics simulation studies. J Comput Chem 42:1736–1749. https://doi.org/10.1002/jcc.26712
Pinzi L, Rastelli G (2019) Molecular docking: Shifting paradigms in drug discovery. Int J Mol Sci 20
Güven ÖÖ, Tahtacı H, Altanlar N, Alıcı H (2022) Synthesis, characterization, antimicrobial activity and in silico studies of some phenyl, furyl and 1H–1,2,4-triazole substituted benzyl and alkyl ethers. ChemistrySelect 7:e202202046. https://doi.org/10.1002/slct.202202046
Ozcan I, Akkoc S, Alici H et al (2023) Novel thioether-bridged 2,6-disubstituted and 2,5,6-trisubstituted imidazothiadiazole analogues: synthesis, antiproliferative activity, ADME, and molecular docking studies. Chem Biodivers 20:e202200884. https://doi.org/10.1002/cbdv.202200884
Yilmaz O, Capanlar S, Akkoc S et al (2022) Design, synthesis, characterization, antiproliferative activity, and in silico studies of novel alkyl ether derivatives containing 1H–1,2,4-triazole ring. ChemistrySelect 7:e202203604. https://doi.org/10.1002/slct.202203604
Das A, Greco G, Kumar S et al (2022) Synthesis, in vitro cytotoxicity, molecular docking and ADME study of some indolin-2-one linked 1,2,3-triazole derivatives. Comput Biol Chem 97:107641. https://doi.org/10.1016/j.compbiolchem.2022.107641
Speck RR, Hildreth J, Flexner C (2002) Differential effects of P-glycoprotein and multidrug resistance protein–1 on productive human immunodeficiency virus infection. J Infect Dis 186:332–340. https://doi.org/10.1086/341464
Husain A, Makadia V, Valicherla GR et al (2022) Approaches to minimize the effects of P-glycoprotein in drug transport: a review. Drug Dev Res 83:825–841. https://doi.org/10.1002/DDR.21918
Karthika C, Sureshkumar R, Zehravi M, et al (2022) Multidrug resistance of cancer cells and the vital role of P-glycoprotein. Life 12:. https://doi.org/10.3390/LIFE12060897
Callaghan R, Luk F, Bebawy M (2014) Inhibition of the multidrug resistance P-glycoprotein: time for a change of strategy? Drug Metab Dispos 42:623. https://doi.org/10.1124/DMD.113.056176
Daura X, Jaun B, Seebach D et al (1998) Reversible peptide folding in solution by molecular dynamics simulation. J Mol Biol 280:925–932
Dong YW, Liao ML, Meng XL, Somero GN (2018) Structural flexibility and protein adaptation to temperature: molecular dynamics analysis of malate dehydrogenases of marine molluscs. Proc Natl Acad Sci U S A 115:1274–1279. https://doi.org/10.1073/PNAS.1718910115/-/DCSUPPLEMENTAL
Jahmidi-Azizi N, Oliva R, Gault S, et al (2021) The effects of temperature and pressure on protein-ligand binding in the presence of mars-relevant salts †. Biology (Basel) 10:. https://doi.org/10.3390/biology10070687
Demir K, Alıcı H, Yaşar F (2018) Conformational stability of the tetrameric de novo designed hexcoil-Ala helical bundle. Chin J Phys 56:46–57. https://doi.org/10.1016/j.cjph.2017.12.004
Author information
Authors and Affiliations
Contributions
Sanket Rathod: conceptualization methodology, software, formal analysis, investigation, resources, data curation, writing (original draft), and visualization. Pooja Chavan: software and writing (original draft). Deepak Mahuli: writing (review and editing); Sneha Rochlani: formal analysis and writing (review and editing). Shalini Shinde: formal analysis and data curation. Swaranjali Pawar: formal analysis and data curation. Prafulla Choudhari: conceptualization, methodology, writing (review and editing), and supervision; Rakesh Dhavale: conceptualization, validation, writing (review and editing), and supervision. Pralhad Mudalkar: writing (review and editing). Firoj Tamboli: writing (review and editing).
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rathod, S., Chavan, P., Mahuli, D. et al. Exploring biogenic chalcones as DprE1 inhibitors for antitubercular activity via in silico approach. J Mol Model 29, 113 (2023). https://doi.org/10.1007/s00894-023-05521-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05521-8