Abstract
Context
The massive emission of carbon dioxide in the world causes global warming and a series of increasingly serious ecological problems. It is urgent to find efficient adsorbent for large-scale CO2 capture. Graphene as a solid adsorbent has exhibited great potential and development prospects in gas adsorption. Doping atoms at defect sites in composite graphene is considered as one of the promising approaches to enhance the gas adsorption ability. Nevertheless, composite graphene doping with different atoms has not been explored to a large extent so far.
Methods
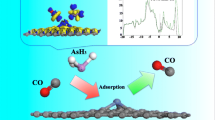
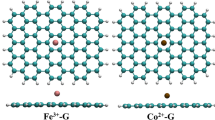
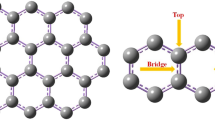
In this work, vacancy graphenes with single C-vacancy (VI-G) and with double C-vacancies (VII-G) are doped with nitrogen atoms and metal atoms M (M = Co, Mo, Mn, Fe) to form composite configurations. The Perdew-Burke-Ernzerho (PBE) functional is used under the generalized gradient approximation (GGA) basis set. A comprehensive study of the adsorption effect and charge transfer characteristics of CO2 molecule on different composite graphene configurations is carried out through DFT calculation. By analyzing the adsorption energy, adsorption distance, energy band structure, and atomic Mulliken population, it is found that the composite graphene doped with metal atoms such as Co-3N-VI, Mo-3N-VI, Mn-3N-VI, Fe-3N-VI, and Mo-4N-VII significantly enhanced the CO2 adsorption. Further analysis of charge density and density of states (DOS) demonstrates that CO2 adsorption on M-3N-VI and M-4N-VII reached the same conclusion. Thus, it is concluded that appropriate metal atoms can enhance the adsorption characteristics.

















Similar content being viewed by others
References
Liu Z, Deng Z, Davis SJ, Giron C, Ciais P (2022) Monitoring global carbon emissions in 2021. Nat Rev Earth Environ 3:217–219
Bongaarts J (2019) Special report on global warming of 1.5 degrees C. Popul Dev Rev 45:251–252
Sun LL, Cui HJ, Ge QS (2022) Will China achieve its 2060 carbon neutral commitment from the provincial perspective? Adv Clim Chang Res 13:169–178
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669
Zhang SD, Wang HH, Liu JP, Bao CL (2020) Measuring the specific surface area of monolayer graphene oxide in water. Mater Lett 261:3
Yu W, Xie HQ, Wang XP, Wang XW (2011) Significant thermal conductivity enhancement for nanofluids containing graphene nanosheets. Phys Lett A 375:1323–1328
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6:183–191
Satyapal S, Petrovic J, Read C, Thomas G, Ordaz G (2007) The US Department of Energy’s National Hydrogen Storage Project: Progress towards meeting hydrogen-powered vehicle requirements. Catal Today 120:246–256
Huang CW, Wu HC, Li YY (2007) Hydrogen storage in platelet graphite nanofibers. Sep Purif Technol 58:219–223
Partoens B, Peeters FM (2006) From graphene to graphite: Electronic structure around the K point. Phys Rev B 74:11
Iyakutti K, Pearses YSM, Suthan T, Surya VJ (2013) Interaction of Hydrogen, Oxygen and BH3 with Graphene-A First Principles Study. Asian J Chem 25:S436–S438
Yang B, Li DB, Qi L, Li TB, Yang P (2019) Thermal properties of triangle nitrogen-doped graphene nanoribbons. Phys Lett A 383:1306–1311
Dai JY, Yuan JM, Giannozzi P (2009) Gas adsorption on graphene doped with B, N, Al, and S: A theoretical study. Appl Phys Lett 95:3
L. Ouyang, J.F. Xiao, H.S. Jiang, S.J. Yuan, Nitrogen-doped porous carbon materials derived from graphene oxide/melamine resin composites for CO2 adsorption, Molecules, 26 (2021) 14.
Nasehnia F, Seifi M (2014) Adsorption of molecular oxygen on VIIIB transition metal-doped graphene: A DFT study. Mod Phys Lett B 28:12
Zhang HY, Tian WJ, Qian Z, Ouyang TH, Saunders M, Qin JY, Wang SB, Tade MO, Sun HQ (2018) Co@C/CoOx coupled with N-doped layer-structured carbons for excellent CO2 capture and oxygen reduction reaction. Carbon 133:306–315
Kim G, Jung SC, Han YK (2013) Selectively strong molecular adsorption on boron nitride monolayer induced by transition metal substrate. Curr Appl Phys 13:2059–2063
Ma L, Zhang JM, Xu KW, Ji V (2015) A first-principles study on gas sensing properties of graphene and Pd-doped graphene. Appl Surf Sci 343:121–127
Wehling T, Novoselov K, Morozov S, Vdovin E, Katsnelson M, Geim A, Lichtenstein A (2008) Molecular doping of graphene. Nano Lett 8:173–177
Zhou QX, Ju WW, Su XY, Yong YL, Li XH (2017) Adsorption behavior of SO2 on vacancy-defected graphene: A DFT study. J Phys Chem Solids 109:40–45
Zhang YH, Chen YB, Zhou KG, Liu CH, Zeng J, Zhang HL, Peng Y (2009) Improving gas sensing properties of graphene by introducing dopants and defects: a first-principles study. Nanotechnology 20:8
Sanyal B, Eriksson O, Jansson U, Grennberg H (2009) Molecular adsorption in graphene with divacancy defects. Phys Rev B 79:4
Panchokarla LS, Subrahmanyam KS, Saha SK, Govindaraj A, Krishnamurthy HR, Waghmare UV, Rao CNR (2009) Synthesis, structure, and properties of boron- and nitrogen-doped graphene. Adv Mater 21:4726-+
Wei DC, Liu YQ, Wang Y, Zhang HL, Huang LP, Yu G (2009) Synthesis of N-doped graphene by chemical vapor deposition and its electrical properties. Nano Lett 9:1752–1758
Wang F, Xu X, Mao J (2020) Can graphene with intrinsic defects electrocatalyze N-2 to NH3 reduction? Diam Relat Mater 109:9
Qin YX, Zhang Z (2020) Co-modulation of graphene by N hetero-doping &vacancy defects and the effect on NO2 adsorption and sensing: First-principles study. Phys E 116:7
Wanno B, Tabtimsai C (2014) A DFT investigation of CO adsorption on VIIIB transition metal-doped graphene sheets. Superlattice Microst 67:110–117
Wang CR, Fang YH, Duan HM, Liang GF, Li WY, Chen DF, Long MJ (2021) DFT study of CO2 adsorption properties on pristine, vacancy and doped graphenes. Solid State Commun 337:10
Clark SJ, Segall MD, Pickard CJ, Hasnip PJ, Probert MI, Refson K, Payne MC (2005) First principles methods using CASTEP. Zeitschrift für kristallographie-crystalline materials 220:567–570
Shamim SUD, Roy D, Alam S, Piya AA, Rahman MS, Hossain MK, Ahmed F (2022) Doubly doped graphene as gas sensing materials for oxygen-containing gas molecules: a first-principles investigation. Appl Surf Sci 596:14
Shukri MSM, Saimin MNS, Yaakob MK, Yahya MZA, Taib MFM (2019) Structural and electronic properties of CO and NO gas molecules on Pd-doped vacancy graphene: a first principles study. Appl Surf Sci 494:817–828
Basiuk VA, Henao-Holguín LV (2014) Dispersion-corrected density functional theory calculations of meso-tetraphenylporphine-C60 complex by using DMol3 module. J Comput Theor Nanosci 11:1609–1615
B. Delley, Hardness conserving semilocal pseudopotentials, Phys Rev B, 66 (2002) 9.
Dolui K, Rungger I, Das Pemmaraju C, Sanvito S (2013) Possible doping strategies for MoS2 monolayers: An ab initio study. Phys Rev B 88:9
S. Kattel, P. Atanassov, B. Kiefer, Stability, electronic and magnetic properties of in-plane defects in graphene: a first-principles study, J Phys Chem C, 116 (2012) 8161-8166.
Hu PB, Wang SJ, Zhuo YQ (2022) CO2 adsorption enhancement over alkaline metal-promoted MgO with SO2, O-2, and H2O present: A theoretical study. Sep Purif Technol 284:17
Nayebi P, Zaminpayma E, Emami-Razavi M (2020) Study of graphene device electronic properties with horizontal and vertical double vacancy defects. Fuller Nanotub Carbon Nanostruct 28:886–890
Tworzydlo J, Trauzettel B, Titov M, Rycerz A, Beenakker CWJ (2006) Sub-Poissonian shot noise in graphene. Phys Rev Lett 96:4
Liu HT, Liu YQ, Zhu DB (2011) Chemical doping of graphene. J Mater Chem 21:3335–3345
Luo GX, Liu LZ, Zhang JF, Li GB, Wang BL, Zhao JJ (2013) Hole Defects and Nitrogen Doping in Graphene: Implication for Supercapacitor Applications. ACS Appl Mater Interfaces 5:11184–11193
Arkhipova EA, Ivanov AS, Maslakov KI, Savilov SV (2020) Nitrogen doping of mesoporous graphene nanoflakes as a way to enhance their electrochemical performance in ionic liquid-based supercapacitors. J Energy Storage 30:8
Guinea F, Katsnelson MI, Geim AK (2010) Energy gaps and a zero-field quantum Hall effect in graphene by strain engineering. Nat Phys 6:30–33
Zhang SJ, Liang Z, Li KJ, Zhang JL, Ren S (2022) A density functional theory study on the adsorption reaction mechanism of double CO2 on the surface of graphene defects. J Mol Model 28:11
Pakornchote T, Ektarawong A, Alling B, Pinsook U, Tancharakorn S, Busayaporn W, Bovornratanaraks T (2019) Phase stabilities and vibrational analysis of hydrogenated diamondized bilayer graphenes: a first principles investigation. Carbon 146:468–475
Ahangari MG, Mashhadzadeh AH, Fathalian M, Dadrasi A, Rostamiyan Y, Mallahi A (2019) Effect of various defects on mechanical and electronic properties of zinc oxide graphene-like structure: a DFT study. Vacuum 165:26–34
Acknowledgements
The authors wish to express their sincere gratitude for the funding supports.
Funding
This work is supported by the National Natural Science Foundation of China (Grant No. 52006029), the Promotion Foundation for Young Science and Technology Talents in Jilin Province (Grant No. QT202113), the Special Foundation of Industrial Innovation in Jilin Province (Grant No. 2019C056-2), and the Special Foundation for Outstanding Young Talents Training in Jilin (Grant No. 20200104107).
Author information
Authors and Affiliations
Contributions
YN conceived and planned the present study. LZY carried out the adsorption simulations of the composite graphene configurations. YN explained the simulation results. LZY arranged the associated images and wrote the first draft of the manuscript. YN and ZYL provided critical feedback and helped to draft the manuscript and supervised the entire study. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Our study is concerned with the molecular simulation of doped composite graphene. We confirm that the manuscript has been read and approved by all named authors. We have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, N., Liu, Z. & Zhou, Y. CO2 adsorption enhancement and charge transfer characteristics for composite graphene doped with atoms at defect sites. J Mol Model 29, 60 (2023). https://doi.org/10.1007/s00894-023-05464-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05464-0