Abstract
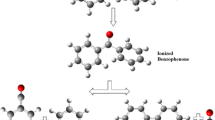
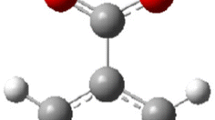
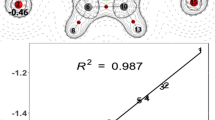
The possibility of finding the fragmentation routes by theoretical methods led us to compare the molecular ions between neutral molecules of benzene, aniline, and o-, m-, and p-nitroaniline, using the density functional theory (DFT), under an aug-cc-pVDZ base set and a B3LYP exchange-correlation functional. After determining the structure and electronic energy of neutral and doubly ionized species, we used a new protocol based on analyzing Wiberg’s binding indexes and the quantum theory of atoms in Bader molecules (QTAIM). The charge transfer and electronic distribution in aromatic monomers indicate the possibility of fragment formation in at least two pairs of carbon-carbon (CC) atoms. They show the possible loss of the -CNH2 and -NO2 groups in the aniline and nitroaniline molecules doubly ionized.
Graphical abstract





Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- oNA:
-
ortho-Nitroanilines
- mNA:
-
meta-Nitroanilines
- pNA:
-
para-Nitroanilines
- BZ:
-
Benzene
- ANI:
-
Aniline
- NA:
-
Nitroanilines
References
Rosner SD, Cameron R, Scholl TJ, Holt RA (1998) A Study of theX2Σ+andA2Π states of SiO+ using fast-ion-beam laser spectroscopy. J Mol Spectrosc 189:83–94. https://doi.org/10.1006/jmsp.1997.7522
Guerra ACO, Ferreira GB, Machado SP, Turci CC (2008) Inner-shell photoabsorption spectroscopy of push–pull nitroanilines—theoretical and experimental studies at N 1s region. Int J Quantum Chem 108:2340–2357. https://doi.org/10.1002/qua.21618
Bartkowiak W, Misiaszek T (2000) Solvent effect on static vibrational and electronic contribution of first-order hyperpolarizability of π-conjugated push–pull molecules: quantum-chemical calculations. Chem Phys 261:353–357. https://doi.org/10.1016/S0301-0104(00)00262-7
Alagia M, Candori P, Falcinelli S, Mundim MSP, Pirani F, Richter R, Rosi M, Stranges S, Vecchiocattivi F (2011) Dissociative double photoionization of singly deuterated benzene molecules in the 26–33 eV energy range. J Chem Phys 135:144304–144308. https://doi.org/10.1063/1.3646516
Pauling L (1932) The nature of the chemical bond. Application of results obtained from the quantum mechanics and from a theory of paramagnetic susceptibility to the structure of molecules. J Chem Phys 1:56–59
Candori P, Falcinelli S, Pirani F, Tarantelli F, Vecchiocattivi F (2007) Interaction components in the hydrogen halide dications. Chem Phys Lett 436:322–326. https://doi.org/10.1016/j.cplett.2007.01.061
Alagia M, Brunetti BG, Candori P, Falcinelli S, Teixidor MM, Pirani F, Richter R, Stranges S, Vecchiocattivi F (2004) Low-lying electronic states of HBr2+. J Chem Phys 120:6985–6991. https://doi.org/10.1063/1.1669383
Prasad SS, Furman DR (1975) On the importance of doubly charged ions in the auroral ionosphere. J Geophys Res Atmosp 80:1360–1362. https://doi.org/10.1029/JA080i010p01360
Kryachko ES (2007) Dicationic states of benzene dimer: benzene dimer cation and benzene dication parenthood pattern. Int J Quantum Chem 107:2741–2755. https://doi.org/10.1002/qua.21432
Tanaka J (1963) The electronic spectra of aromatic molecular crystals. I. Substitued benzene molecules. Bull Chem Soc Jpn 36:833–847. https://doi.org/10.1246/bcsj.36.833
Khalil OS, McGylnn SP (1975) Electronic spectroscopy of highly-polar aromatics. XIII. absorption and luminescence of nitroanilines. J Lumin 11:185–196. https://doi.org/10.1016/0022-2313(75)90013-7
Bertinelli F, Palmieri P, Brillante A, Taliani C (1977) Electronic-excited states of nitroanilines. II. A configuration interaction study and UV spectrum of the paranitroaniline single. Crystal Chem Phys 25:333–341. https://doi.org/10.1016/0301-0104(77)85143-4
Mebel AM, Lin SH, Yang XM, Lee YT (1997) Theoretical study on the mechanism of the dissociation of benzene. The C5H3 + CH3 product channel. J Phys Chem A 101:6781–6789. https://doi.org/10.1021/jp970596l
Klippenstein SJ, Faulk JD, Dunbar RC (1993) A combined theoretical and experimental study of the dissociation of benzene cation. J Chem Phys 98:243–256. https://doi.org/10.1063/1.464670
Blanksby SJ, Ellison GB (2003) Bond dissociation energies of organic molecules. Acc Chem Res 36:255–263. https://doi.org/10.1021/ar020230d
Gu Q, Knee JL (2008) Binding energies and dissociation pathways in the aniline-Ar2 cation complex. J Chem Phys 128:1–8. https://doi.org/10.1063/1.2827458
Russo N, Toscano M, Grand A, Mineva T (2000) Proton affinity and protonation sites of aniline. Energetic behavior and density functional reactivity índices. J Phys Chem A 140:4017–4021. https://doi.org/10.1021/jp991949e
Choe JC, Cheong NR, Park SM (2009) Unimolecular dissociation of aniline molecular ion: a theoretical study. Int J Mass Spectrom 279:25–31. https://doi.org/10.1016/j.ijms.2008.09.013
Bandyopadhyay G, Mondal R, Lahiri SC (2001) Dissociation constants of p-nitroaniline in mixed solvents and a critical assessment of the solvent-sorting equilibrium method for the determination of the single ion Gibbs energy of transfer of H+ ion from aqueous to aquo rich organic solvents. Zeitschrift Für Phys Chemie 215:13–28. https://doi.org/10.1524/zpch.2001.215.1.013
Cabral BJC (2013) Electron binding energies and the fundamental gap of a push-pull dye in a polar environment: p-nitroaniline in liquid water. Chem Phys letters 138:234304–234308. https://doi.org/10.1016/j.cplett.2016.11.017
Oliveira CX, Mocellin A, Lima FMS, Neto AMJC, Azevedo DL (2020) DFT study of L-cysteine fragmentation route using a novel protocol. Chemistry Select 5:439–447. https://doi.org/10.1002/slct.201903453
Wiberg KB (1968) Application of the pople-santry-segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 14:1083–1096. https://doi.org/10.1016/0040-4020(68)88057-3
Bader RFW (1994) Atoms in molecules: a quantum theory. Clarendon Press, Oxford
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Petersson, G.A., Nakatsuji, H., Li, X., Caricato, M., Marenich, A.V., Bloino, J., Janesko, B. G., Gomperts, R., Mennucci, B., Hratchian, H.P., Ortiz, J.V., Izmaylov, A.F., Sonnenberg, J.L., Williams-Young, D., Ding, F., Lipparini, F., Egidi, F., Goings, J., Peng, B., Petrone, A., Henderson, T., Ranasinghe, D., Zakrzewski, V.G., Gao, J., Rega, N., Zheng, G., Liang, W., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Throssell, K., Montgomery, J A., Jr., Peralta, J.E., Ogliaro, F., Bearpark, M.J., Heyd, J.J., Brothers, E.N., Kudin, K.N., Staroverov, V.N., Keith, T. A., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A.P., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Millam, J. M., Klene, M., Adamo, C., Cammi, R., Ochterski, J.W., Martin, R.L., Morokuma, K., Farkas, O., Foresman, J. B., Fox, D.J.: Gaussian 09 Revision E.01, Inc., Wallingford CT (2016).
Hohenberg P, Kohn W (1964) Inhomogeneous electron gas. Phys Rev 136:B864–B871. https://doi.org/10.1103/PhysRev.136.B864
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140:A1133–A1138. https://doi.org/10.1103/PhysRev.140.A1133
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Dunning Jr TH (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys 90:1007–1023. https://doi.org/10.1063/1.456153
Breneman CM, Wiberg KB (1990) Determining atom-centered monopoles from molecular electrostatic potentials. The need for high sampling density in formamide conformational analysis. J Comput Chem 11:361–373. https://doi.org/10.1002/jcc.540110311
Kumar PSV, Raghavendra V, Subramanian V (2016) Bader’s theory of atoms in molecules (AIM) and its applications to chemical bonding. J Chem Sci 128:1527–1536. https://doi.org/10.1007/s12039-016-1172-3
Keith, T.A.: AIMAll (Version 14.11.23). TK Gristmill Software, Overland Park KS, USA, (2019) Available: http://aim.tkgristmill.com
Chemissian v4.51 released| Chemissian: software to analyze spectra, and build density maps and molecular orbitals (2017). Available: https://www.chemissian.com
Wasylishen R, Rowbotham JB, Ernst L, Schaefer T (1972) Long-range spin–spin coupling constants from amino protons and 15N to ring protons in aniline-15N and some derivatives. INDO Molecular Orbital Calculations. Can J Chem 50:2575–2585. https://doi.org/10.1139/v72-414
Baba M, Kowaka Y, Nagashima U, Ishimoto T, Goto H, Nakayama N (2011) Geometrical structure of benzene and naphthalene: ultrahigh-resolution laser spectroscopy and ab initio calculation. J Chem Phys 135:054305-11-054305-5. https://doi.org/10.1063/1.3622766
Trivedi MK, Branton A (2015) Isotopic abundance analysis of biofield treated benzene, toluene and p-xylene using gas chromatography-mass spectrometry (GC-MS). Mass Spectrom Purif Tech 1:1–6
Reilly JP, Kompa KL (1980) Laser induced multiphoton ionization mass spectrum of benzene. J Chem Phys 73:5468–5476
Jennings KR (1965) Metastable transitions in the mass spectrum of benzene. J Chem Phys 43:4176–4177
Jennings KR (1967) The decomposition of benzene and deuterated benzenes under electron impact. Zeitschrift Fur Naturforsch 22:454–459
Reeher JR, Flesch GD, Svec HJ (1976) The mass spectra and ionization potentials of the neutral fragments produced during the electron bombardment of aromatic compounds. Org Mass Spectrom 11:154–166
Wojciechowski PM, Zierkiewicz W, Michalska D, Hobza P (2003) Electronic structures, vibrational spectra, and revised assignment of aniline and its radical cation: theoretical study. J Chem Phys 118:10900–10911
King GA, Oliver TAA, Ashfold MNR (2010) Dynamical insights into 𝜋1𝜎∗ state mediated photodissociation of aniline. J Chem Phys 132:214307-1–214307-11
Lalli PM, Iglesias BA, Toma HE, De Sa GF, Daroda RJ, Filho JCS, Szulejko JE, Araki K, Eberlin MN (2012) Protomers: formation, separation and characterization via travelling wave ion mobility mass spectrometry. J Mass Spectrom 47:712–719
Nicolescu TO (2017) interpretation of mass spectra, In mass spectrometry, edited by Mahmood Aliofkhazraei. IntechOpen, London
Ploug-Sørensen G, Andersen EK (1983) Structure of o-nitroaniline hydrochloride, C6H7N2O2+. Cl−, Acta Crystallogr Sect C Cryst.Struct Commun 39:112–114
Azhagiri S, Ramkumaar GR, Jayakumar S, Kumaresan S, Arunbalaji R, Gunasekaran S, Srinivasan S (2010) Theoretical and experimental studies of vibrational spectra and thermal analysis of 2-nitroaniline and its cation. J Mol Model 16:87–94
Maihub AA, Alassbaly FS, El-Ajaily MM, Etorki AM (2014) Modification on synthesis of mixed ligand chelates by using Di- and trivalent transition metal ions with schiff base as primary ligand, green sustain. Chem 4:103–110
Linstrom PJPJ, Mallard WGG (2001) The NIST chemistry webbook: a chemical data resource on the internet. J Chem Eng Data 46:1059–1063
Wu Guo-Hua ZY-W, Liu-Si S, Hui G (1997) Photoionization studies of m-nitroaniline using synchrotron radiation. Acta Phys-Chim Sin 13:317–321
Kumar Trivedi M, Branton A (2015) Impact of biofield treatment on spectroscopic and physicochemical properties of p-nitroaniline, Insights Anal Electrochem 1:1–8
Barros VP, Assis MD (2013) Iron porphyrins as biomimetical models for disperse azo dye oxidation. J Braz Chem Soc 24:830–836
Matsumoto A, Suzuki M, Hayashi H, Kuzuhara D, Yuasa J, Kawai T, Aratani N, Yamada H (2016) Aromaticity relocation in perylene derivatives upon two-electron oxidation to form anthracene and phenanthrene. Chem- A Eur J 22:14462–14466
Lewars EG (2011) The concept of the potential energy surface. computational chemistry. Springer, Cham
Caramori GF, De Oliveira KT (2009) Aromaticidade: evolução histórica do conceito e critérios quantitativos. Quim Nova 32:1871–1884
Lu T, Chen F (2013) Bond order analysis based on the Laplacian of electron density in fuzzy overlap space. J Phys Chem A 117:3100–3108
Gillespie RJ, Matta CF (2001) Teaching the VSEPR model and electron densities. Chem Educ Res Pr 2:73–90
J.W. Ochterski, Thermochemistry in Gaussian, Gaussian Inc Pittsburgh PA (2000).
Gurvich LV (1989) Reference books and data banks on the thermodynamic properties of individual substances. Pure Appl Chem 61:1027–1031
Chase Jr MW, Davies CA, Downey Jr JR, Frurip DJ, McDonald RA (1985) JANAF Thermochemical Tables Third Edition. J Phys Chem Ref Data Monogr or Suppl 14
B. Ruscic, Active Thermochemical Tables (ATcT) values based on ver. 1.118 of the Thermochemical Network (2015); available at ATcT.anl.gov, (2015).
Ruscic B, Pinzon RE, Morton ML, Von Laszevski G, Bittner SJ, Nijsure SG, Amin KA, Minkoff M, Wagner AF (2004) Introduction to active thermochemical tables: Several “key” enthalpies of formation revisited. J Phys Chem A 108:9979–9997
Curtiss LA, Redfern PC, Raghavachari K, Pople JA (1998) Assessment of Gaussian-2 and density functional theories for the computation of ionization potentials and electron affinities. J Chem Phys 109:42–55
Bentley TW, Wellington CA (1981) Doubly charged benzene and isomeric dications. Fragmentation energetics and charge distributions calculated by MINDO/3 molecular orbital theory. Org Mass Spectrom 16:523–526
Koopmans T (1934) Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den einzelnen Elektronen eines Atoms. Physica 1:104–113
Liao SC (1970) Dipole moments, charge-transfer parameters, and ionization potentials of the methyl-substituted benzene–tetracyanoethylene complexes. Can J Chem 48:299–305
Oudar JL, Chemla DS (1977) Hyperpolarizabilities of the nitroanilines and their relations to the excited state dipole moment. J Chem Phys 66:2664–2668
Thander A, Mallik B (2000) Charge-transfer spectra of ferrocene in halocarbon solvents under photoexcitation. J Chem Sci 112:475–485
Meek JT, Sekreta E, Wilson W, Viswanathan KS, Reilly JP (1985) The laser photoelectron spectrum of gas phase aniline. J Chem Phys 82:1741–1749
Khalil OS, Meeks JL, McGlynn SP (1973) Electronic spectroscopy of highly polar aromatics. VII. Photoelectron spectra of nitroanilines. J Am Chem Soc 95:5876–5880
Johnstone RAW, Mellon FA (1973) Effects of induction and resonance in the calculation of ionization potentials of substituted benzenes by perturbation molecular orbital theory. J Chem Soc Faraday Trans 2(69):36–42
Acknowledgements
This work has been financially supported by CAPES for providing post-graduate scholarship and the Institute of Physics, University of Brasília (UnB). In memory of Dra. Maria Suely P. Mundin (UnB).
Code availability
Not applicable.
Author information
Authors and Affiliations
Contributions
Ms. Carlos Xavier de Oliveira: conceptualization, investigation, methodology, visualization, writing-original draft. Dr. Fabio L P Costa: data curation, writing-review editing. Dr. Gunar V S Mota: visualization, writing-review editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All the authors gave their consent for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oliveira, C.X., Costa, F.L. & Mota, G.V.S. Fragmentation route of doubly ionized benzene, aniline, and nitroanilines monomers using a novel protocol from density functional theory and QTAIM. J Mol Model 29, 53 (2023). https://doi.org/10.1007/s00894-023-05461-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05461-3