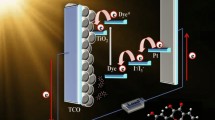
Abstract
Context
The improvement of new organic flavone-based donor-spacer-acceptor (D-π-A) type dye molecules of the 3-(4-hydroxypiperidin-2-yloxy)-7-hydroxy-2-(3,4-dihydroxyphenyl)-4H-chromen-4-one (D1), 7-hydroxy-2-(3,4-dihydroxyphenyl)-3-(piperidin-4-yloxy)-4H-chromen-4-one (D2), and 3-((2-aminopyridin-4-yloxy)methoxy)-7-hydroxy-2-(3,4-dihydroxyphenyl)-4H-chromen-4-one (D3) were successfully designed and synthesized for dye-sensitized solar cells (DSSCs).
Methods
Here, we discuss the synthesis of flavone compounds as well as their photophysical and electrochemical characterization. Using the Gaussian 09w software, the electronic structures and apsorption spectra have been calculated at the B3LYP, B3PW91, CAM-B3LYP, MPW1PW91, PBEPBE, and ωB97XD theory with the 6-311G(d,p) basis sets.
Results
The computed values of the D2 molecule ground state optimized HOMOs-LUMOs energy is well positioned for advantageous charge transfer (CT) into the semiconducting material (TiO2) as well as the electron injection process. With a high power conversion efficiency (PCE) of 3.46% (VOC = 0.718 V, JSC = 7.07 mA cm−2, and FF = 0.68), the D2 compound also demonstrated good photovoltaic (PV) properties.
Conclusion
These findings unequivocally demonstrate that altering the D-π-A metal-free organic material electron-withdrawing capacity is a useful strategy for enhancing the optical and electrical characteristics of the organic PV system.










Similar content being viewed by others
Data availability
All the data and electronic materials available for Gaussian program.
References
Nozik AJ, Miller J (2010) Introduction to solar photon conversion. Chem Rev 110(11):6443–6445
Brédas JL, Norton JE, Cornil J, Coropceanu V (2009) Molecular understanding of organic solar cells: the challenges. Acc Chem Res 42(11):1691–1699
Graetzel M, Janssen RA, Mitzi DB, Sargent EH (2012) Materials interface engineering for solution-processed photovoltaics. Nature 488(7411):304–312
O’regan B, Grätzel M (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353(6346):737–740
Peic A, Staff D, Risbridger T, Menges B, Peter LM, Walker AB, Cameron PJ (2011) Real-time optical waveguide measurements of dye adsorption into nanocrystalline TiO2 films with relevance to dye-sensitized solar cells. J Phys Chem C 115(3):613–619
Ning Z, Fu Y, Tian H (2010) Energy Environ Sci 3:1170
Wang P, Zakeeruddin SM, Exnar I, Grätzel M (2002) High efficiency dye-sensitized nanocrystalline solar cells based on ionic liquid polymer gel electrolyte. Chem Commun 24:2972–2973
De Angelis F, Fantacci S, Selloni A, Nazeeruddin MK, Grätzel M (2010) First-principles modeling of the adsorption geometry and electronic structure of Ru (II) dyes on extended TiO2 substrates for dye-sensitized solar cell applications. J Phys Chem C 114(13):6054–6061
Wang ZS, Cui Y, Hara K, Dan-oh Y, Kasada C, Shinpo A (2007) A high-light-harvesting-efficiency coumarin dye for stable dye-sensitized solar cells. Adv Mater 19(8):1138–1141
Hara K, Kurashige M, Dan-oh Y, Kasada C, Shinpo A, Suga S, Sayama K, Arakawa H (2003) Design of new coumarin dyes having thiophene moieties for highly efficient organic-dye-sensitized solar cells. New J Chem 27(5):783–785
Liu B, Zhu W, Zhang Q, Wu W, Xu M, Ning Z, Xie Y, Tian H (2009) Conveniently synthesized isophorone dyes for high efficiency dye-sensitized solar cells: tuning photovoltaic performance by structural modification of donor group in donor-π-acceptor system. Chem Commun 13:1766–1768
Zafer C, Kus M, Turkmen G, Dincalp H, Demic S, Kuban B, Teoman Y, Icli S (2007) New perylene derivative dyes for dye-sensitized solar cells. Sol Energy Mater Sol Cells 91(5):427–431
Ma X, Hua J, Wu W, Jin Y, Meng F, Zhan W, Tian H (2008) A high-efficiency cyanine dye for dye-sensitized solar cells. Tetrahedron 64(2):345–350
Hayashi S, Tanaka M, Hayashi H, Eu S, Umeyama T, Matano Y, Araki Y, Imahori H (2008) Naphthyl-fused π-elongated porphyrins for dye-sensitized TiO2 cells. J Phys Chem C 112(39):15576–15585
Shen P, Liu Y, Huang X, Zhao B, Xiang N, Fei J, Liu L, Wang X, Huang H, Tan S (2009) Efficient triphenylamine dyes for solar cells: effects of alkyl-substituents and π-conjugated thiophene unit. Dyes Pigm 83(2):187–197
Liu D, Zhao B, Shen P, Huang H, Liu L, Tan S (2009) Molecular design of organic dyes based on vinylene hexylthiophene bridge for dye-sensitized solar cells. Sci China Ser B Chem 52(8):1198–1209
Pei K, Wu Y, Wu W, Zhang Q, Chen B, Tian H, Zhu W (2012) Constructing organic D-A-π-A-featured sensitizers with a quinoxaline unit for high-efficiency solar cells: the effect of an auxiliary acceptor on the absorption and the energy level alignment. Chem A Euro J 18(26):8190–8200
Li X, Cui S, Wang D, Zhou Y, Zhou H, Hu Y, Liu JG, Long Y, Wu W, Hua J, Tian H (2014) New organic donor-acceptor-π-acceptor sensitizers for efficient dye-sensitized solar cells and photocatalytic hydrogen evolution under visible-light irradiation. Chemsuschem 7(10):2879–2888
Chang DW, Lee HJ, Kim JH, Park SY, Park SM, Dai L, Baek JB (2011) Novel quinoxaline-based organic sensitizers for dye-sensitized solar cells. Org Lett 13(15):3880–3883
Cui Y, Wu Y, Lu X, Zhang X, Zhou G, Miapeh FB, Zhu W, Wang ZS (2011) Incorporating benzotriazole moiety to construct D-A-π-A organic sensitizers for solar cells: significant enhancement of open-circuit photovoltage with long alkyl group. Chem Mater 23(19):4394–4401
Liu J, Liu B, Tang Y, Zhang W, Wu W, Xie Y, Zhu WH (2015) Highly efficient cosensitization of D-A-π-A benzotriazole organic dyes with porphyrin for panchromatic dye-sensitized solar cells. J Mater Chem C 3(42):11144–11150
Hu X, Cai S, Tian G, Li X, Su J, Li J (2013) Rigid triarylamine-based D-A-π-A structural organic sensitizers for solar cells: the significant enhancement of open-circuit photovoltage with a long alkyl group. RSC Adv 3(44):22544–22553
Li W, Wu Y, Zhang Q, Tian H, Zhu W (2012) D-A-π-A featured sensitizers bearing phthalimide and benzotriazole as auxiliary acceptor: effect on absorption and charge recombination dynamics in dye-sensitized solar cells. ACS Appl Mater Interfaces 4(3):1822–1830
Hendsbee AD, McAfee SM, Sun JP, McCormick TM, Hill IG, Welch GC (2015) Phthalimide-based π-conjugated small molecules with tailored electronic energy levels for use as acceptors in organic solar cells. J Mater Chem C 3(34):8904–8915
Huang ZS, Cai C, Zang XF, Iqbal Z, Zeng H, Kuang DB, Wang L, Meier H, Cao D (2015) Effect of the linkage location in double branched organic dyes on the photovoltaic performance of DSSCs. J Mater Chem A 3(3):1333–1344
Labat F, Ciofini I, Hratchian HP, Frisch M, Raghavachari K, Adamo C (2009) First principles modeling of eosin-loaded ZnO films: a step toward the understanding of dye-sensitized solar cell performances. J Am Chem Soc 131(40):14290–14298
Jacquemin D, Perpete EA, Ciofini I, Adamo C (2009) Accurate simulation of optical properties in dyes. Acc Chem Res 42(2):326–334
Barone V, Polimeno A (2007) Integrated computational strategies for UV/vis spectra of large molecules in solution. Chem Soc Rev 36(11):1724–1731
Jamorski Jödicke C, Lüthi HP (2003) Time-dependent density functional theory (TDDFT) study of the excited charge-transfer state formation of a series of aromatic donor-acceptor systems. J Am Chem Soc 125(1):252–264
Jodicke CJ, Luthi HP (2002) Time-dependent density-functional theory investigation of the formation of the charge transfer excited state for a series of aromatic donor-acceptor systems. Part II J Chem Phys 117(9):4157–4167
Preat J, Jacquemin D, Wathelet V, André JM, Perpète EA (2006) TD-DFT investigation of the UV spectra of pyranone derivatives. J Phys Chem A 110(26):8144–8150
Gómez-Ortíz NM, Vázquez-Maldonado IA, Pérez-Espadas AR, Mena-Rejón GJ, Azamar-Barrios JA, Oskam G (2010) Dye-sensitized solar cells with natural dyes extracted from achiote seeds. Sol Energy Mater Sol Cells 94(1):40–44
Huamán AA, Celestino MR, Quintana ME (2021) Theoretical and experimental study of solar cells based on nanostructured films of TiO2 sensitized with natural dyes extracted from Zea mays and Bixa orellana. RSC Adv 11(16):9086–9097
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H (2009) Revision D. 01, Gaussian Inc. Wallingford CT.
Becke AD (2014) Perspective: fifty years of density-functional theory in chemical physics. J Chem Phys 140(18):18A301
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37(2):785
Ren X, Li J, Holmes RJ, Djurovich PI, Forrest SR, Thompson ME (2004) Ultrahigh energy gap hosts in deep blue organic electrophosphorescent devices. Chem Mater 16(23):4743–4747
Fan C, Wei Y, Ding D, Xu H (2015) Linkage engineering in hosts for dramatic efficiency enhancement of blue phosphorescent organic light-emitting diodes. Opt Exp 23(10):12887–12899
Daeneke T, Kwon TH, Holmes AB, Duffy NW, Bach U, Spiccia L (2011) High-efficiency dye-sensitized solar cells with ferrocene-based electrolytes. Nature Chem 3(3):211–215
Bisquert J (2010) Theory of the impedance of charge transfer via surface states in dye-sensitized solar cells. J Electroanal Chem 646(1–2):43–51
Pearson RG (1986) Absolute electronegativity and hardness correlated with molecular orbital theory. Proc Natl Acad Sci 83(22):8440–8441
Solomon RV, Veerapandian P, Vedha SA, Venuvanalingam P (2012) Tuning nonlinear optical and optoelectronic properties of vinyl coupled triazene chromophores: a density functional theory and time-dependent density functional theory investigation. J Phys Chem A 116(18):4667–4677
Gajalakshmi D, Solomon RV, Tamilmani V, Boobalan M, Venuvanalingam P (2015) A DFT/TDDFT mission to probe push-pull vinyl coupled thiophene oligomers for optoelectronic applications. RSC Adv 5(62):50353–50364
Gece G (2008) The use of quantum chemical methods in corrosion inhibitor studies. Corros Sci 50(11):2981–2992
Parr RG, Szentpály LV, Liu S (1999) Electrophilicity index. J Am Chem Soc 121(9):1922–1924
Chattaraj PK, Maiti B, Sarkar U (2003) Philicity: a unified treatment of chemical reactivity and selectivity. J Phys Chem A 107(25):4973–4975
Parr RG, Donnelly RA, Levy M, Palke WE (1978) Electronegativity: the density functional viewpoint. J Chem Phys 68(8):3801–3807
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105(26):7512–7516
Marinado T, Nonomura K, Nissfolk J, Karlsson MK, Hagberg DP, Sun L, Mori S, Hagfeldt A (2010) How the nature of triphenylamine-polyene dyes in dye-sensitized solar cells affects the open-circuit voltage and electron lifetimes. Langmuir 26(4):2592–2598
Rühle S, Greenshtein M, Chen SG, Merson A, Pizem H, Sukenik CS, Cahen D, Zaban A (2005) Molecular adjustment of the electronic properties of nanoporous electrodes in dye-sensitized solar cells. J Phys Chem B 09(40):18907–18913
Zhang J, Li HB, Sun SL, Geng Y, Wu Y, Su ZM (2012) Density functional theory characterization and design of high-performance diarylamine-fluorene dyes with different π spacers for dye-sensitized solar cells. J Mater Chem 22(2):568–576
Preat J, Jacquemin D, Michaux C, Perpète EA (2010) Improvement of the efficiency of thiophene-bridged compounds for dye-sensitized solar cells. Chem Phys 376(1–3):56–68
Islam A, Sugihara H, Arakawa H (2003) Molecular design of ruthenium (II) polypyridyl photosensitizers for efficient nanocrystalline TiO2 solar cells. J Photochem Photobiol A Chem 158(2–3):131–138
Katoh R, Furube A, Yoshihara T, Hara K, Fujihashi G, Takano S, Murata S, Arakawa H, Tachiya M (2004) Efficiencies of electron injection from excited N3 dye into nanocrystalline semiconductor (ZrO2, TiO2, ZnO, Nb2O5, SnO2, In2O3) films. J Phys Chem B 108(15):4818–4822
Ning Z, Zhang Q, Wu W, Pei H, Liu BO, Tian HE (2008) Starburst triarylamine based dyes for efficient dye-sensitized solar cells. J Org Chem 73(10):3791–3797
Daeneke T, Mozer AJ, Uemura Y, Makuta S, Fekete M, Tachibana Y, Koumura N, Bach U, Spiccia L (2012) Dye regeneration kinetics in dye-sensitized solar cells. J Am Chem Soc 134(41):16925–16928
Robson KC, Hu K, Meyer GJ, Berlinguette CP (2013) Atomic level resolution of dye regeneration in the dye-sensitized solar cell. J Am Chem Soc 135(5):1961–1971
Liu Y, Yang G, Sun S, Su Z (2012) Density functional theory investigation on the second-order nonlinear optical properties of chlorobenzyl-o-carborane derivatives. Chinese J Chem 30(10):2349–2355
Funding
The authors extend their appreciation to the Research Center for Advanced Materials Science (RCAMS), King Khalid University, Saudi Arabia, for funding this work under grant Number: RCAMS/KKU/020–22.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors (Arunkumar Ammasi, Ragavan Iruthayaraj, Anbarasan Ponnusamy Munusamy, and Mohd Shkir).
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This is not applicable.
Consent for publication
This is not applicable.
Competing interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ammasi, A., Iruthayaraj, R., Munusamy, A.P. et al. Molecular engineering on D-π-A organic dyes with flavone-based different acceptors for highly efficient dye-sensitized solar cells using experimental and computational study. J Mol Model 29, 45 (2023). https://doi.org/10.1007/s00894-023-05445-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05445-3