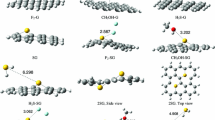
Abstract
Context
The reactivity of graphene oxide (GO) with amines is related to the ring-opening of the epoxy groups in its basal surface, as addressed experimentally. Therefore, discussing the hydroxyl/epoxy ratio for GO is relevant to improve the characterization of such material. As the adsorption of Methylene Blue into GO is related to a graphene derivative’s oxidation degree (OD), we combined adsorption experimental information and theoretical data to estimate the hydroxyl/epoxy ratio. The theoretical data were compared to the experimental adsorption of Methylene Blue and Indigo Carmine into GO synthesized in our department. Our results show GO systems with hydroxyl/epoxy ratios equal to 0.7, 0.8, and 0.9 are the most representative in which the most coherent model corresponds to OH/EP=0.8 for our GO previously synthesized.
Methods
The GO-MODEL software was developed in the present work to obtain cartesian coordinates of GO systems. We investigated 204 systems comprising models with 486 carbon atoms with the GFN2-xTB semiempirical quantum method. The supramolecular arrangements were constructed with the recently developed UD-APARM program.
Graphical Abstract






Similar content being viewed by others
Data availability
Starting GO structures (XYZ) obtained through the GO-MODEL software and Supporting Information file comprising: Data for isotherm study (MB/GO); Langmuir parameters for MB/GO system; Data for isotherm study (IC/GO); Langmuir parameters for IC/GO system; Structure information of the GO models; Supramolecular systems for α-Euler = 0 and 1800, and UD-APARM input parameters employed.
References
Zhu BY et al (2010) Graphene and graphene oxide: synthesis, properties, and applications. Adv Mater 22(35):3906–3924. https://doi.org/10.1002/adma.201001068
A Razaq, F Bibi, X Zheng, R Papadakis, S H M Jafri, H. Li (2022) Review on graphene-, graphene oxide-, reduced graphene oxide-based flexible composites: from fabrication to applications,” Materials (Basel) 15 (3). https://doi.org/10.3390/ma15031012.
Dreyer DR, Jia H-P, Bielawski CW (2010) Graphene oxide: a convenient carbocatalyst for facilitating oxidation and hydration reactions. Angew Chemie 122(38):6965–6968. https://doi.org/10.1002/ange.201002160
Mohamadi S, Naderian A, Nazari B (2022) Evaluation of different organic solvents adsorption by porous ammonium-treated graphene and graphene oxide sponges. Chem Pap 76(4):2537–2548. https://doi.org/10.1007/s11696-021-02050-7
Hadi M, Mollaei T (2019) Reduced graphene oxide/graphene oxide hybrid-modified electrode for electrochemical sensing of tobramycin. Chem Pap 73(2):291–299. https://doi.org/10.1007/s11696-018-0578-4
Molla A, Li Y, Mandal B, Kang SG, Hur SH, Chung JS (2019) Selective adsorption of organic dyes on graphene oxide: theoretical and experimental analysis. Appl Surf Sci 464(June 2018):170–177. https://doi.org/10.1016/j.apsusc.2018.09.056
Tang H et al (2018) Wrinkle- and edge-adsorption of aromatic compounds on graphene oxide as revealed by atomic force microscopy, molecular dynamics simulation, and density functional theory. Environ Sci Technol 52(14):7689–7697. https://doi.org/10.1021/acs.est.8b00585
Tang H et al (2018) Theoretical insight into the adsorption of aromatic compounds on graphene oxide. Environ Sci Nano 5(10):2357–2367. https://doi.org/10.1039/c8en00384j
TavassoliLarijani H, Darvish Ganji M, Jahanshahi M (2015) Trends of amino acid adsorption onto graphene and graphene oxide surfaces: a dispersion corrected DFT study”. RSC Adv 5(113):92843–92857. https://doi.org/10.1039/c5ra16683g
Boukhvalov DW, Katsnelson MI (2008) Modeling of graphite oxide. J Am Chem Soc 130(32):10697–10701. https://doi.org/10.1021/ja8021686
Gómez-Navarro C et al (2010) Atomic structure of reduced graphene oxide. Nano Lett 10(4):1144–1148. https://doi.org/10.1021/nl9031617
Savazzi F, Risplendi F, Mallia G, Harrison NM, Cicero G (2018) Unravelling some of the structure-property relationships in graphene oxide at low degree of oxidation. J Phys Chem Lett 9(7):1746–1749. https://doi.org/10.1021/acs.jpclett.8b00421
Dave SH, Gong C, Robertson AW, Warner JH, Grossman JC (2016) Chemistry and structure of graphene oxide via direct imaging. ACS Nano 10(8):7515–7522. https://doi.org/10.1021/acsnano.6b02391
Georgakilas V et al (2012) Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications. Chem Rev 112(11):6156–6214. https://doi.org/10.1021/cr3000412
Vacchi IA, Spinato C, Raya J, Bianco A, Ménard-Moyon C (2016) Chemical reactivity of graphene oxide towards amines elucidated by solid-state NMR. Nanoscale 8(28):13714–13721. https://doi.org/10.1039/c6nr03846h
Kasprzak A, Zuchowska A, Poplawska M (2018) Functionalization of graphene: does the organic chemistry matter. Beilstein J Org Chem 14(1):2018–2026. https://doi.org/10.3762/bjoc.14.177
Smith M, Scudiero L, Espinal J, McEwen JS, Garcia-Perez M (2016) Improving the deconvolution and interpretation of XPS spectra from chars by ab initio calculations. Carbon N Y 110:155–171. https://doi.org/10.1016/j.carbon.2016.09.012
A Rawal, S H C Man, V Agarwal, Y Yao, S C Thickett, P B Zetterlund (2021) Structural complexity of graphene oxide: the Kirigami model. https://doi.org/10.1021/acsami.1c01157.
Liu W, Speranza G (2021) Tuning the oxygen content of reduced graphene oxide and effects on its properties. ACS Omega 6(9):6195–6205. https://doi.org/10.1021/acsomega.0c05578
Al-Gaashani R, Najjar A, Zakaria Y, Mansour S, Atieh MA (2019) XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram Int 45(11):14439–14448. https://doi.org/10.1016/j.ceramint.2019.04.165
Yan H et al (2014) Effects of the oxidation degree of graphene oxide on the adsorption of methylene blue. J Hazard Mater 268:191–198. https://doi.org/10.1016/j.jhazmat.2014.01.015
Grimme S, Bannwarth C, Shushkov P (2017) A robust and accurate tight-binding quantum chemical method for structures, vibrational frequencies, and noncovalent interactions of large molecular systems parametrized for all spd-block elements (Z = 1–86). J Chem Theory Comput 13(5):1989–2009. https://doi.org/10.1021/acs.jctc.7b00118
Bannwarth C, Ehlert S, Grimme S (2019) GFN2-xTB - an accurate and broadly parametrized self-consistent tight-binding quantum chemical method with multipole electrostatics and density-dependent dispersion contributions. J Chem Theory Comput 15(3):1652–1671. https://doi.org/10.1021/acs.jctc.8b01176
Ehlert S, Stahn M, Spicher S, Grimme S (2021) Robust and efficient implicit solvation model for fast semiempirical methods. J Chem Theory Comput 17(7):4250–4261. https://doi.org/10.1021/acs.jctc.1c00471
Anconi CPA (2020) Relative position and relative rotation in supramolecular systems through the analysis of the principal axes of inertia: ferrocene/cucurbit[7]uril and ferrocenyl azide/β-cyclodextrin case studies. ACS Omega 5(10):5013–5025. https://doi.org/10.1021/acsomega.9b03914
Kim S et al (2021) PubChem in 2021: new data content and improved web interfaces. Nucleic Acids Res 49(D1):D1388–D1395. https://doi.org/10.1093/nar/gkaa971
El-Mansy MAM (2017) Quantum chemical studies on structural, vibrational, nonlinear optical properties and chemical reactivity of indigo carmine dye. Spectrochim. Acta - Part A Mol. Biomol Spectrosc 183:284–290
A. Of, M. Blue, and O. F. S. Areas (1960) Adsorption of methylene blue in (4)
Y. Yukselen, M. Asce, A. Kaya, and M. Asce (2006) Comparison of methods for determining specific surface area of soils 132 (7) 931–936
Hegyesi N, Vad RT, Pukánszky B (2017) Applied clay science determination of the speci fi c surface area of layered silicates by methylene blue adsorption : the role of structure, pH and layer charge. Appl Clay Sci 146(January):50–55. https://doi.org/10.1016/j.clay.2017.05.007
P. Montes-navajas, N. G. Asenjo, and A. Corma (2013) Surface area measurement of graphene oxide in aqueous solutions.
Z. Benzait and L. Trabzon (2022) Graphite size effect on chemical expansion and graphene oxide properties. https://doi.org/10.1021/acsomega.2c05059.
Alkhouzaam A, Qiblawey H, Khraisheh M, Atieh M, Al-Ghouti M (2020) Synthesis of graphene oxides particle of high oxidation degree using a modified Hummers method. Ceram Int 46(15):23997–24007
G. Santamaría-Juárez, E. Gómez-Barojas, E. Quiroga-González, E. Sánchez-Mora, M. Quintana-Ruiz, and J. D. Santamaría-Juárez (2019) Safer modified Hummers’ method for the synthesis of graphene oxide with high quality and high yield. Mater Res Express 6 (12). https://doi.org/10.1088/2053-1591/ab4cbf
Justh N, Berke B, László K, Szilágyi IM (2018) Thermal analysis of the improved Hummers’ synthesis of graphene oxide. J Therm Anal Calorim 131(3):2267–2272. https://doi.org/10.1007/s10973-017-6697-2
Morimoto N et al (2017) Real-time, in situ monitoring of the oxidation of graphite: lessons learned. Chem Mater 29(5):2150–2156. https://doi.org/10.1021/acs.chemmater.6b04807
Kekes T, Tzia C (2020) Adsorption of indigo carmine on functional chitosan and β-cyclodextrin/chitosan beads: equilibrium, kinetics and mechanism studies. J. Environ. Manage 262(February):110372. https://doi.org/10.1016/j.jenvman.2020.110372
Shi H, Li W, Zhong L, Xu C (2014) Methylene blue adsorption from aqueous solution by magnetic cellulose/graphene oxide composite: equilibrium, kinetics, and thermodynamics. Ind Eng Chem Res 53(3):1108–1118. https://doi.org/10.1021/ie4027154
Li Y et al (2013) Methylene blue adsorption on graphene oxide/calcium alginate composites. Carbohydr Polym 95(1):501–507. https://doi.org/10.1016/j.carbpol.2013.01.094
Han R, Zhang J, Han P, Wang Y, Zhao Z, Tang M (2009) Study of equilibrium, kinetic and thermodynamic parameters about methylene blue adsorption onto natural zeolite. Chem Eng J 145(3):496–504. https://doi.org/10.1016/j.cej.2008.05.003
Milonjić SK (2007) A consideration of the correct calculation of thermodynamic parameters of adsorption. J Serbian Chem Soc 72(12):1363–1367. https://doi.org/10.2298/JSC0712363M
Ghosal PS, Gupta AK (2017) Determination of thermodynamic parameters from Langmuir isotherm constant-revisited. J Mol Liq 225:137–146. https://doi.org/10.1016/j.molliq.2016.11.058
Lima EC, Hosseini-Bandegharaei A, Moreno-Piraján JC, Anastopoulos I (2019) A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J Mol Liq 273:425–434. https://doi.org/10.1016/j.molliq.2018.10.048
Karaçetin G, Sivrikaya S, Imamoʇlu M (2014) Adsorption of methylene blue from aqueous solutions by activated carbon prepared from hazelnut husk using zinc chloride. J Anal Appl Pyrolysis 110(1):270–276. https://doi.org/10.1016/j.jaap.2014.09.006
Thangavel S, Venugopal G (2014) Understanding the adsorption property of graphene-oxide with different degrees of oxidation levels. Powder Technol 257:141–148. https://doi.org/10.1016/j.powtec.2014.02.046
Harrache Z, Abbas M, Aksil T, Trari M (2019) Thermodynamic and kinetics studies on adsorption of Indigo Carmine from aqueous solution by activated carbon. Microchem. J. 144(2018):180–189. https://doi.org/10.1016/j.microc.2018.09.004
M. J. Mcallister et al (2007) Expansion of graphite (4)4396–4404
Z. Li, C Wang (2010) Intercalation of Methylene Blue in a high-charge calcium montmorillonite — an indication of surface charge determination 3 (1) 297–312
Acknowledgements
Larissa Cristina Aparecida Souza thanks the Brazilian agency Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarship. The authors also thank Professor Hélio Ferreira dos Santos for the access to the NEQC (Núcleo de Estudos em Química Computacional, UFJF, Brazil) computer facility and the Laboratório Central de Computação Científica (LCC) da UFLA for providing additional computing resources. Finally, we also thank Sthephano Daniel Santos for the adapted GO models included in the Supplementary Information.
Funding
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) supported this study.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the design and implementation of the research and revised the results. Specifically, Regis Vinicius Alves de Abreu and Mário César Guerreiro are responsible for the experimental data. Furthermore, Cleber Anconi developed and wrote the GO-MODEL software in Fortran language, supervised the research, and wrote the manuscript along with inputs from the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This is not applicable
Consent for publication
This is not applicable.
Competing interest
The authors declare no competing interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Souza, L.C.A., Abreu, R.V.A., Guerreiro, M.C. et al. Estimating hydroxyl/epoxy ratio in graphene oxide through adsorption experiment and semiempirical GFN2-xTB quantum method. J Mol Model 29, 42 (2023). https://doi.org/10.1007/s00894-023-05444-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05444-4