Abstract
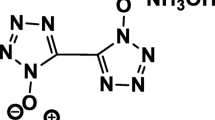
TKX-50 (dihydroxylammonium 5,5′-bistetrazolate-1,1′-dioxide) is a recent time invention by Klapotke et. al. in the field of high energy materials, and it outperforms all the existing materials by means of performance parameters. It is rising as potential energetic material due to favorable thermal insensitivity, low toxicity and safe handling. The decomposition temperature (Tmax) values of precursors such as glyoxime (I), 1,2-dichloroglyoxime (II), 1,2-diazidoglyoxime (III), and bistetrazoledihydroxide (IV) and ending products TKX-50 (V) and ABTOX (VI) have been attempted to correlate with the results obtained from molecular electrostatic potentials and band gaps calculated from the difference of ionization potential and electron affinity. The molecular electrostatic potential values of azido attached -NO group of III are much less than that of hydro/chloro attached -NO group of I/II and that of tetrazole groups IV, V, and VI. The band gaps calculated from stability trend in the increasing order of III < II < I < IV < V < VI are well corroborated with stability trend drawn from experimentally determined decomposition temperatures. Further, employing conceptual density functional theory (DFT) molecular descriptors, band gap values were calculated via the difference of ionization potential and electron affinity to understand the thermal stability of TKX-50, ABTOX, and its precursors.

Similar content being viewed by others
Data availability
The online version of this article contains supplementary material, which is available to authorized users. It contains absolute electronic energies and Cartesian coordinates of all the optimized stationary points at wb97xd/6–311 + + g(3df,3pd) in the gas phase including corresponding charge and multiplicity.
References
Talawar MB, Nandagopal S, Singh S, Mahajan AP, Badgujar DM, Khan GM, MAS, (2018) TGA, DSC and DFT studies of TKX-50, ABTOX and their key precursors. ChemistrySelect 23:12175–12182
Xiao L-B, Zhao F-Q, Luo Y, Li N, Gao H-X, Xue Y-Q, Cui Z-X, Hu R-Z (2016) Thermal behavior and safety of dihydroxylammonium 5,50-bistetrazole-1,10-diolate. J Therm Anal Calorim 123:653–657
Yuan B, Yu Z, Bernstein ER (2015) Initial mechanisms for the decomposition of electronically excited energetic salts: TKX-50 and MAD-X1. J Phys Chem A 119:2965–2981
Dreger ZA, Tao Y, Averkiev BB, Gupta YM, Klapötke TM (2015) High pressure stability of energetic crystal of dihydroxylammonium 5,5’-bistetrazole-1,1’-diolate (TKX-50): Raman spectroscopy and DFT calculations. J Phys Chem B 119:6836–6847
An Q, Cheng T, Goddard WA III, Zybin SV (2015) Anisotropic impact sensitivity and shock induced plasticity of TKX-50 (dihydroxylammonium 5,5’-bistetrazole-1,1’-diolate) single crystals: from large-scale molecular-dynamics simulations. J Phys Chem C 119(4):2196–2207
Huang H, Shi Y, Yang J (2015) Thermal characterization of the promising energetic material TKX-50. J Therm Anal Calorim 121:705–709
An Q, Liu W-G, Goddard WA III, Cheng T, Zybin SV, Xiao H (2014) Initial steps of thermal decomposition of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate crystals from quantum mechanics. J Phys Chem C 118:27175–27181
Niu H, Chen S, Jin S, Li L, Jing B, Jiang Z, Ji J, Shu Q (2016) Thermolysis, nonisothermal decomposition kinetics, calculated detonation velocity and safety assessment of dihydroxylammonium 5, 50-bistetrazole-1, 10-diolate. J Therm Anal Calorim 126:473–480
Yu Y, Chen S, Li T, Jin S, Zhang G, Chenb M, Li L (2017) Study on a novel high energetic and insensitive munitions formulation: TKX-50 based melt cast high explosive. RSC Adv 7:31485–31492
Chai J-D, Martin H-G (2008) Systematic optimization of long-range corrected hybrid density functionals. J Chem Phys 128:084106
Chai J-D, Martin H-G (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620
Khan MAS, Lo R, Bandyopadhyay T, Ganguly B (2011) Probing the reactivation process of sarin-inhibited acetylcholinesterase with α-nucleophiles: hydroxylamine anion is predicted to be a better antidote with DFT calculations. J Mol Graphics Modell 29:1039–1046
Khan MAS, Ganguly B (2012) Assessing the reactivation efficacy of hydroxylamine anion towards VX-inhibited AChE: a computational study. J Mol Model 18:1801–1808
Khan MAS, Bandyopadhyay T, Ganguly B (2012) Assessing the reactivation efficacy of hydroxylamine anion towards VX-inhibited AChE: a computational study. J Mol Graphics Modell 34:10–17
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JRJ, Montgomery Jr., A, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2010) Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT.
Politzer P, Murra JS, Lipkowitz KB, Boyd DB (1991) Molecular electrostatic potentials and chemical reactivity, Reviews in Computational Chem., 2:273–312. Chapter 7, Wiley-VCH Publishers, New York, Print ISBN: 9780471188100.
Khan MAS, Sen A, Ganguly B (2009) Probing the influence of pH dependent citric acid towards the morphology of rock salt: a computational study. Cryst Eng Comm 11:2660–2667
Khan MAS, Singh A, Haldar S, Ganguly B (2011) Can nitrilotriacetic acid (NTA) act as a habit modifier for rock salt crystals? An answer from computational and experimental studies, Cryst Growth Des 11:1675–1682
Shee SK, Reddy ST, Athar J, Sikder AK, Talawar MB, Banerjee S, Khan MAS (2015) Probing the compatibility of energetic binder poly-glycidyl nitrate with energetic plasticizers: thermal, rheological and DFT studies. RSC Adv 5:101297–101308
Khan MAS, Zhang J, Sarma KD, Ganguly B (2012) Origins of reversing diastereoselectivity of α, β-dichloro-γ-butenolides and γ-butyrolactams in direct vinylogous aldol addition: a computational study. RSC Adv 2:8460–8466
Fischer N, Fischer D, Klapötke TM, Piercey DG, Stierstorfer J (2012) Pushing the limits of energetic materials – the synthesis and characterization of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate. J Mater Chem 22:20418–20422
Acknowledgements
The authors thank the director of HEMRL, Pune, for his constant encouragement to undertake work on TKX-50.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception, design data collection, and analysis. The first draft of the manuscript was written by Md Abdul Shafeeuulla Khan, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adak, P.K., Singh, S.K., Singh, J. et al. A DFT approach towards understanding the thermal stability of TKX-50 and their key precursors through band gaps and MESP. J Mol Model 28, 400 (2022). https://doi.org/10.1007/s00894-022-05398-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05398-z