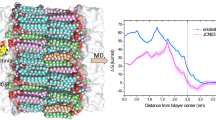
Abstract
AZD3759 is an epidermal growth factor receptor inhibitor with good blood–brain barrier permeability, demonstrating encouraging activity against central nervous system metastases. However, the underlying mechanism was still unclear. In this study, the interaction between AZD3759 and membrane was studied with 1,2-dimyristoyl-sn-glycero-3-phosphocholine bilayer as a model lipid. Both the cationic and neutral state of AZD3759 were considered in the simulations, and the results show that cationic AZD3759 forms more hydrogen bonds with bilayer than neutral AZD3759, and Coulombic interaction has great effects in the transmembrane process of cationic AZD3759. AZD3759 prefers to reside in the interface between the hydrophilic headgroup region and hydrophobic region of bilayer, and the chloroflurobenzene moiety plays a crucial role in the insertion of AZD3759. PMF results suggest that the hydrophobic region of DMPC bilayer is permeable by AZD3759. Understanding the transmembrane mechanism of AZD3759 at molecular level may provide useful information to the design and optimization of anti-tumor drugs with improved BBB penetration.
Graphical Abstract
• The penetration mechanism of AZD3759 with DMPC bilayer was studied by molecular dynamics simulations.
• Neutral AZD3759 could penetrate deeper into DMPC bilayer than protonated AZD3759.
• The chloroflurobenzene moiety plays a significant role in the insertion of AZD3759 into DMPC bilayer.
• The electrostatic interaction is the driving force for the initial binding of AZD3759 to DMPC bilayer.
• Our findings may enhance the mechanism understanding of drugs with good BBB permeability.











Similar content being viewed by others
Data availability
All relevant data are within the paper and its supporting information file.
References
Kwatra MM (2017) A rational approach to target the epidermal growth factor receptor in glioblastoma. Curr Cancer Drug Targets 17(3):290–296
Chistiakov DA, Chekhonin IV, Chekhonin VP (2017) The EGFR variant III mutant as a target for immunotherapy of glioblastoma multiforme. Eur J Pharmacol 810:70–82
Seystahl K, Gramatzki D, Roth P, Weller M (2016) Pharmacotherapies for the treatment of glioblastoma–current evidence and perspectives. Expert Opin Pharmaco 17(9):1259–1270
Shi T, Wang L, Fan Z, Feng L, Wang Y (2020) Specific organ metastases and survival of advanced non-small cell lung cancer (NSCLC) patients with EGFR mutation. J Clinical Oncology 38(15_suppl):e21527
Zeng Q, Wang J, Cheng Z, Chen K, Johnström P, Varnäs K, Li DY, Yang ZF, Zhang X (2015) Discovery and evaluation of clinical candidate AZD3759, a potent, oral active, central nervous system-penetrant, epidermal growth factor receptor tyrosine kinase inhibitor. J Med Chem 58(20):8200–8215
Heffron TP (2016) Small molecular kinase inhibitors for the treatment of brain cancer. J Med Chem 59:10030–10066
Ahn MJ, Kim DW, Kim TM et al (2016) Phase I study of AZD3759, a CNS penetrable EGFR inhibitor, for the treatment of non-small-cell lung cancer (NSCLC) with brain metastasis (BM) and leptomeningeal metastasis (LM). J Clin Oncol 34(15_suppl):9003
Remon J, Steuer CE, Ramalingam SS, Felip E (2018) Osimertinib and other third-generation EGFR TKI in EGFR-mutant NSCLC patients. Ann Oncol 29(suppl 1):i20–i27
Ahn MJ, Kim DW, Cho BC et al (2017) Activity and safety of AZD3759 in EGFR-mutant non-small-cell lung cancer with CNS metastases (BLOOM): a phase 1, open-label, dose-escalation and dose-expansion study. Lancet Respir Med 5(11):891–902
Yang Z, Guo Q, Wang Y et al (2016) AZD3759, a BBB-penetrating EGFR inhibitor for the treatment of EGFR mutant NSCLC with CNS metastases. Sci Transl Med 8(368):368ra172
Li X, Wang Y, Wang J, Zhang T, Zheng L, Yang Z, Xing L, Yu J (2018) Enhanced efficacy of AZD3759 and radiation on brain metastasis from EGFR mutant non-small cell lung cancer. Int J Cancer 143:212–224
Xiong B, Wang Y, Chen Y, Xiang S, Liao Q, Chen Y, Li Q, Li W, Sun H (2021) Strategies for structural modification of small molecules to improve blood-brain barrier penetration: a recent perspective. J Med Chem 64(18):13152–13173
Mensch J, Melis A, Mackie C, Verreck G, Brewster ME, Augustijns P (2010) Evaluation of various PAMPA models to identity the most discriminating method for the prediction of BBB permeability. Eur J Pharm Biopharm 74:495–502
Gupta M, Lee HJ, Barden CJ, Weaver DF (2019) The blood-brain barrier (BBB) score. J Med Chem 62:9824–9836
Stepnik K, Kukula-Koch W (2020) In silico studies on triterpenoid saponins permeation through the blood-brain barrier combined with postmortem research on the brain tissues of mice affected by Astragaloside IV administration. Int J Mol Sci 21:2534
Liu L, Zhang L, Feng H, Li S, Liu M, Zhao J, Liu H (2021) Prediction of the blood-brain barrier (BBB) permeability of chemicals based on machine-learning and ensemble method. Chem Res Toxicol 34(6):1456–1467
Garg P, Verma J (2006) In silico prediction of blood brain barrier permeability: an artificial neutral network model. J Chem Inf Model 46:289
Carpenter TS, Kirshner DA, Lau EY, Wong SE, Nilmeier JP, Lightstone FC (2014) A method to predict blood-brain barrier permeability of drug-like compounds using molecular dynamics simulations. Biophys J 107:630–641
Lee CT, Comer J, Herndon C, Leung N, Pavlova A, Swift RV, Tung C, Rowley CN, Amaro RE, Chipot C, Wang Y, Gumbart JC (2016) Simulation-based approaches for determining membrane permeability of small compounds. J Chem Inf Model 56:721–733
Vermaas JV, Dixon RA, Chen F, Mansfield SD, Boerjan W, Ralph J, Crowley MF, Beckham GT (2019) Passive membrane transport of lignin-related compounds. Proc Natl Acad Sci USA 116:23117–23123
Siwy CM, Delfing BM, Smith AK, Klimov DK (2020) Partitioning of benzoic acid into 1,2-dimyristoyl-sn-glycero-3-phosphocholine and blood-brain barrier mimetic bilayers. J Chem Inf Model 60(8):4030–4046
Thai NQ, Theodorakis PE, Li MS (2020) Fast estimation of the blood-brain barrier permeability by pulling a ligand through a lpid membrane. J Chem Inf Model 60:3057–3067
Wang Y, Gallagher E, Jorgensen C, Troendle EP, Hu D, Searson PC, Ulmschneider MB (2019) An experimentally validated approach to calculate the blood-brain barrier permeability of small molecules. Sci Rep 9:6117
Jorgensen C, Ulmschneider MB, Searson PC (2022) Atomistic model of solute transport across the blood-brain barrier. ACS Omega 7(1):1100–1112
Campbell SD, Regina KJ, Kharasch ED (2014) Significance of lipid composition in a blood-brain barrier—mimetic PAMPA Assay. J Biomol Screening 19:437–444
Siakotos AN, Rouser G, Fleischer S (1969) Isolation of highly purified human and bovine brain endothelial cells and nuclei and their phospholipid composition. Lipids 4:234–239
Faulker C, de Leeuw N (2021) Predicting the membrane permeability of fentanyl and its analogues by molecular dynamics simulations. J Phys Chem B 125(30):8443–8449
Jo S, Kim T, Iyer VG, Im W (2008) CHARMM-GUI: a web-based graphical user interface for CHARMM. J Comput Chem 29:1859–1865
Lee J, Cheng X, Swails JM, Yeom MS, Eastman PK, Lemkul JA, Wei S, Buckner J, Jeong JC, Qi Y, Jo S, Pande VS, Case DA, Brooks CL III, MacKerell AD Jr, Klauda JB, Im W (2016) CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM and CHARMM/OpenMM simulations using the CHARMM36 additive force filed. J Chem Theory Comput 12:405–413
Lee J, Patel DS, Ståhle J, Park SJ, Kern NR, Kim S, Lee J, Cheng X, Valvano MA, Holst O, Knirel Y, Qi Y, Jo S, Klauda JB, Widmalm G, Im W (2019) CHARMM-GUI membrane builder for complex biological membrane simulations with glycolipids and lipoglycans. J Chem Theory Comput 15:775–786
Klauda JB, Kučerka N, Brooks BR, Pastor RW, Nagle JF (2006) Simulation-based methods for interpreting X-ray data from lipid bilayers. Biophys J 90:2796–2807
Darden T, York D, Pedersen L (1993) Particle mesh Ewald: AnN·log(N) method for Ewald sums in large systems. J Chem Phys 98:10089–10092
Yu W, He X, Vanommeslaeghe K, MacKerell AD Jr (2012) Extension of the CHARMM general force field to sulfonyl-containing compounds and its utility in biomolecular simulations. J Comput Chem 33(31):2451–2468
Vanommeslaeghe K, Hatcher E, Acharya C, Kundu S, Zhong S, Shim J, Darian E, Guvench O, Lopes P, Vorobyov I, Mackerell AD Jr (2010) CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J Comput Chem 31:671–690
Kumar S, Rosenberg JM, Bouzida D, Swendsen RH, Kollman PA (1992) The weighted histogram analysis method for free-energy calculations on biomolecules: I. The method J Comput Chem 13:1011–1021
Hub JS, de Groot BL, van der Spoel D (2010) g_wham—a free weighted histogram analysis implementation including robust error and autocorrelation estimates. J Chem Theory Comput 6:3713–3720
Hess B, Kutzner C, van der Spoel D, Lindahl E (2008) GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput 4:435–447
Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E (2015) GROMACS: high performance molecular simulations through muti-level parallelism from laptops to supercomputers. SoftwareX 1–2:19–25
Ulander J, Haymet ADJ (2003) Permeation across hydrated DPPC lipid bilayers: Simulation of titrable amphiphilic drug valproic acid. Biophys J 85:3475–3484
Liu W, Zhang S, Meng F, Tang L (2014) Molecular simulation of ibuprofen passing across POPC membrane. J Theor Comput Chem 13(4):1450033
Boggara MB, Krishnamoorti R (2010) Partitioning of nonsteroidal anti-inflammatory drugs in lipid membranes. Biophys J 98:586–595
Pickholz M, Oliveira ON Jr, Skaf MS (2007) Interactions of chlorpromazine with phospholipid monolayers: effects of the ionization state of the drug. Biophys Chem 125(2–3):425–434
Cardenas AE, Shrestha R, Webb LJ, Elber R (2015) Membrane permeation of a peptide: it is better to be positive. J Phys Chem B 119(21):6412–6420
Oruç T, Küçük SE, Sezer D (2016) Lipid bilayer permeation of aliphatic amine and carboxylic acid drugs; rates of insertion, translocation and dissociation from MD simulations. Phys Chem Chem Phys 18(35):24511–24525
Mahar Doan KM, Humphreys JE, Webster LO, Wring SA, Shampine LJ, Serabjit-Singh CJ, Adkison KK, Polli JW (2002) Passive permeability and Pglycoprotein-mediated efflux differentiate central nervous system (CNS) and non-CNS marketed drugs. J Pharmacol Exp Ther 303:1029–1037
Wager TT, Villalobos A, Verhoest PR, Hou X, Shaffer CL (2011) Strategies to optimize the brain availability of central nervous system drug candidates. Expert Opin Drug Discov 6:371–381
Hitchcock SA, Pennington LD (2006) Structure-brain exposure relationships. J Med Chem 49:7559–7583
Ghose AK, Herbertz T, Hudkins RL, Dorsey BD, Mallamo JP (2012) Knowledge based, central nervous system (CNS) lead selection and lead optimization for CNS drug discovery. ACS Chem Neurosci 3:50–68
Rankovic Z (2015) CNS drug design: balancing physicochemical properties for optimal brain exposure. J Med Chem 58:2584–2608
Howell BA, Chauhan A (2009) Interaction of cationic drugs with liposomes. Langmuir 25(20):12056–12065
Anderson CM, Cardenas A, Elber R, Webb LJ (2019) Preferential equilibrium partitioning of positively charged tryptophan into phosphatidylcholine bilayer. J Phys Chem B 123(1):170–179
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 51974201).
Author information
Authors and Affiliations
Contributions
Fancui Meng and Zhixia Qiao conceived and designed the study. The calculations and analysis were performed by Yanshu Liang and Shuang Zhi. All authors contributed to the first draft of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
N/A.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, Y., Zhi, S., Qiao, Z. et al. Molecular dynamics simulations of a central nervous system-penetrant drug AZD3759 with lipid bilayer. J Mol Model 28, 261 (2022). https://doi.org/10.1007/s00894-022-05266-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05266-w