Abstract
Indole compounds are widely found in natural products and drug candidates. The transition-metal–catalyzed regioselective C-H bond functionalization of indoles as the most efficient method for the synthesis of various functionalized indoles has been extensively studied in the past two decades due to its advantages of step economy and atom economy. In general, the catalysts included the transition-metals (Pd, Rh, Ru, Cu, Co, Fe, Zn, and Ga) and these reactions were accomplished with a remarkably wide range of coupling reagents for construction of various C–C and C-X (X = N, O, S) bonds. However, the general and important rules of the regioselectivity are not clear to date. Therefore, a comprehensive analysis through previous reported theoretical studies on transition-metal–catalyzed regioselective C-H bond functionalization of indoles was crucial and significant. In this review, we found that when the C-H bond activation process was the rate-determining step, the regioselectivity ordinarily occurred at the C7 or C4 positions (on benzene ring), and otherwise, the regioselectivity often occurred at C2 position (on pyrrole ring). For indoline substrates, the C-H bond functionalization occurred at the benzene ring.
Graphical abstract
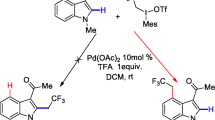
General rules of the regioselectivities for transition-metal-catalyzed C-H bond functionalization of indoles. This review collects major advances in the transition-metal-catalyzed C-H bond functionalization of indoles and indolines










Similar content being viewed by others
Data availability
There is no data available in the public domain related to this work.
Code availability
N/A.
References
Sundberg R-J (1970) The chemistry of indoles. Academic Press, New York
Ban Y, Murakami Y, Iwasawa Y, Tsuchiya M, Takano N (1988) Indole Alkaloids in Medicine. Med Res Rev 8:231–308
Sundberg R-J (1996) Indoles. Academic Press, San Diego
Pindur U, Lemster T (2001) Advances in marine natural products of the indole and annelated indole series: chemical and biological aspects. Curr Med Chem 8:1681–1698
Kochanowska-Karamyan A-J, Hamann M-T (2010) Marine indole alkaloids: potential new drug leads for the control of depression and anxiety. Chem Rev 110:4489–4497
Humphrey G-R, Kuethe J-T (2006) Practical methodologies for the synthesis of indoles. Chem Rev 106:2875–2911
Cacchi S, Fabrizi G (2011) Update 1 of: synthesis and functionalization of indoles through palladium-catalyzed reactions. Chem Rev 111:215–283
Bandini M, Eichholzer A (2009) Catalytic functionalization of indoles in a new dimension. Angew Chem Int Ed 48:9608–9644
Gul W, Hamann M (2005) Indole alkaloid marine natural products: an established source of cancer drug leads with considerable promise for the control of parasitic, neurological and other diseases. Life Sci 78:442–453
Noland WE, Brown CD, DeKruif RD, Lanzatella NP, Gao SM, Zabronsky AE and Tritch KJ (2018) Condensation reactions of indole with acetophenones affording mixtures of 3,3-(1-phenylethane-1,1-diyl)bis(1H-indoles) and 1,2,3,4-tetrahydro-3-(1H-indol-3-yl)-1-methyl-1,3-diphenylcyclopent[b]indoles. Synthetic Communications 48:1755–1765
Sandtorv AH (2015) Transition metal-catalyzed C-H activation of indoles. Adv Synth Catal 357:2403–2435
Ma B et al (2009) Total synthesis of the antimitotic bicyclic peptide celogentin C. Angew Chem Int Ed 48:6104–6107
Yeung CS, Dong VM (2011) Catalytic dehydrogenative cross-coupling: forming carbon−carbon bonds by oxidizing two carbon-hydrogen bonds. Chem Rev 111:1215–1292
Ackermann L (2011) carboxylate-assisted transition-metal-catalyzed c−h bond functionalizations: mechanism and scope. Chem Rev 111:1315–1345
Bras JL, Muzart J (2011) intermolecular dehydrogenative heck reactions. Chem Rev 111:1170–1214
Lyons TW, Sanford MS (2010) Palladium-catalyzed ligand-directed C-H functionalization reactions. Chem Rev 110:1147–1169
Chen X, Engle KM, Wang D-H, Yu J-Q (2009) Palladium(II)-catalyzed C-H activation/C-C cross-coupling reactions: versatility and practicality. Angew Chem Int Ed 48:5094–5115
Lewis JC, Bergman RG, Ellman JA (2008) Direct functionalization of nitrogen heterocycles via Rh-catalyzed C−H bond activation. Acc Chem Res 41:1013–1025
Ding S, Jiao N (2011) Direct transformation of N, N-dimethylformamide to -CN: Pd-catalyzed cyanation of heteroarenes via C-H functionalization. J Am Chem Soc 133:12374–12377
Xu S, Huang X, Hong X, Xu B (2012) Palladium-assisted regioselective C-H cyanation of heteroarenes using isonitrile as cyanide source. Org Lett 14:4614–4617
Ding S, Jiao N (2012) N, N-Dimethylformamide: A Multipurpose Building Block. Angew Chem Int Ed 51:9226–9237
Yu DG et al (2014) Cobalt(III)-Catalyzed Functionalization of unstrained carbon–carbon bonds through β-carbon cleavage of alcohols. J Am Chem Soc 136:17722–17725
Shu Z et al (2014) Iron(II)-Catalyzed direct cyanation of arenes with aryl(cyano)iodonium triflates. Angew Chem Int Ed 53:2186–2189
Yang Y et al (2011) Lewis acid catalyzed direct cyanation of indoles and pyrroles with N-Cyano-N-phenyl-p-toluenesulfonamide (NCTS). Org Lett 13:5608–5611
Reddy BVS et al (2010) Pd(OAc)2-catalyzed C-H activation of indoles: a facile synthesis of 3-cyanoindoles. Tetrahedron Lett 51:3334–3336
Yan G et al (2010) Palladium-catalyzed direct cyanation of indoles with K4[Fe(CN)6]. Org Lett 12:1052–1055
Beckers I, Krasniqi B, Kumar P, Escudero D, De Vos D (2021) Ligand-controlled selectivity in the Pd-catalyzed C−H/C−H cross-coupling of indoles with molecular oxygen. ACS Catal 11:2435–2444
Li J, Ackermann L (2015) Cobalt-catalyzed C-H cyanation of arenes and heteroarenes. Angew Chem Int Ed 54:3635–3638
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396
Ikemoto H et al (2014) Pyrroloindolone synthesis via a Cp*CoIII-catalyzed redox-neutral directed C−H alkenylation/annulation sequence. J Am Chem Soc 136:5424–5431
Kou X et al (2013) Copper-catalyzed aromatic C-H bond cyanation by C-CN bond cleavage of inert acetonitrile. Chem Eur J 19:16880–16886
Cai Y et al (2011) Iron-catalyzed C-H fuctionalization of indoles. Adv Synth Catal 353:2939–2944
Nagase Y, Sugiyama T, Nomiyama S, Yonekura K, Tsuchimoto T (2014) Zinc-catalyzed direct cyanation of indoles and pyrroles: nitromethane as a source of a cyano group. Adv Synth Catal 356:347–352
Pan Z, Liu L, Xu S, Shen Z (2021) Ligand-free iridium-catalyzed regioselective C-H borylation of indoles. RSC Adv 11:5487–5490
Okamoto K et al (2012) Practical synthesis of aromatic nitriles via gallium-catalysed electrophilic cyanation of aromatic C-H bonds. Chem Commun 48:3127–3129
Qiu X, Shi Z et al (2019) PIII-chelation-assisted indole C7-arylation, olefination, methylation, and acylation with carboxylic acids/anhydrides by rhodium catalysis. Angew Chem Int Ed 58:1504–1508
Wang Y, Shi Y, Shao J, Deng C (2021) Theoretical insights into the mechanism of rhodium-catalyzed, PIII-directed regioselective C H arylation of indole with anhydride. Int J Quantum Chem 121:e26475
Gorelsky S, Lapointe D, Fagnou K (2008) Analysis of the concerted metalation-deprotonation mechanism in palladium-catalyzed direct arylation across a broad range of aromatic substrates. J Am Chem Soc 130:10848–10849
Xu L et al (2016) Rhodium-catalyzed regioselective C7-functionalization of N-pivaloylindoles. Angew Chem Int Ed 55:321–325
Han L, Ma X, Liu Y, Yu Z, Liu T (2018) Mechanistic insight into the C7-selective C-H functionalization of N-acyl indole catalyzed by a rhodium complex: a theoretical study. Org Chem Front 5:725–733
Qiu X et al (2018) Rhodium-catalyzed, P-directed selective C7 arylation of indoles. Sci Adv 4:6468
Mu X, Ge X, Zhong X, Han L, Liu T (2020) Mechanistic insight into the rhodium-catalyzed, P-directed selective C7 arylation of indoles: a DFT study. Molecular Catal 495:111147
Borah AJ, Shi Z (2017) Palladium-Catalyzed Regioselective C-H fluoroalkylation of indoles at C4-position. Chem Commun 53:3945–3948
Shi Y, Ma H, Shao J, Deng C (2021) Theoretical studies on the mechanism of Pd2+-catalyzed regioselective C-H alkylation of indole with MesICH2CF3OTf. J Mol Model 27:150
Mishra N-K, Kim I-S et al (2015) Rhodium(III)-Catalyzed selective C-H cyanation of indolinesand indoles with an easily accessible cyano source. Adv Synth Catal 357:1293–1298
Deng C et al (2019) Theoretical studies on Rh(III)-catalyzed regioselective C-H bond cyanation of indole and indoline. Dalton Trans 48:168–175
Wu X, Ji H et al (2018) Ruthenium(II)-Catalyzed regio- and stereoselective C-H Allylation of indoles with allyl alcohols. Org Lett 20:2224–2227
Deng C et al (2019) Theoretical studies on the mechanism of Ru(II)-catalyzed regioselective C-H allylation of indoles with allyl alcohols. Dalton Trans 48:9181–9186
Wang X, Lane BS, Sames D (2005) Direct C-arylation of free (NH)-indoles and pyrroles catalyzed by Ar−Rh(III) complexes assembled in situ. J Am Chem Soc 127:4996–4997
Santoro S, Himo F (2017) Mechanism and selectivity of rhodium-catalyzed C-H bond arylation of indoles Int J Quantum Chem e25526
Wang X et al (2015) Access to six- and seven-membered 1,7-fused indolines via Rh(III)-catalyzed redox-neutral C7-selective C-H functionalization of indolines with alkynes and alkenes. J Org Chem 80:6238–6249
Han L, Zhang X, Wang X, Zhao F, Liu S, Liu T (2017) Mechanistic insights into the selective cyclization of indolines with alkynes and alkenes to produce six- and seven-membered 1,7-fused indolines via Rh(III) catalysis: a theoretical study. Org Biomol Chem 15:3938–3946
Becke AD (1993) Density functional thermochemistry. III. The Role of Exact Exchange. J Chem Phys 98:5648–5652
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241
Hay P-J, Wadt W-R (1985) Ab initio effective core potentials for molecular calculations–Potentials for main group elements Na to Bi. J Chem Phys 82:284–298
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys 7:3297–3305
Funding
This work was supported by the Bioinformatics Center of Nanjing Agricultural University.
Author information
Authors and Affiliations
Contributions
H. Ma: writing original draft; T. Yu, L. Chi: literature collection; C. Huang, X. Li: literature analysis and investigation; R. Zhang: manuscript revision; C. Deng: writing and supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, H., Yu, T., Chi, L. et al. Recent advances in theoretical studies on transition-metal–catalyzed regioselective C-H functionalization of indoles. J Mol Model 28, 267 (2022). https://doi.org/10.1007/s00894-022-05265-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05265-x