Abstract
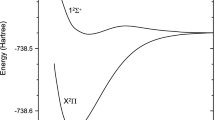
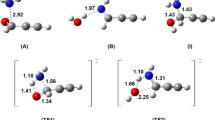
Phosphorus is a key and vital element for a diverse set of important biological molecules, being indispensable for life as we know. A deeper comprehension of its role in astrochemistry and atmospheric chemistry may aid in finding answers to how this element became available on Earth. The PO molecule is one of the main reservoirs of phosphorus in the interstellar medium (ISM), and a better understanding of the mechanisms and rate coefficients for its formation in the ISM is important for modelling its abundances. In this work, we perform multireference configuration interaction calculations on the formation of PO via the \(\mathrm {P}(^{4}S)+\mathrm {O}_{2}(^{3}\Sigma ^{-})\) reaction, analyzing its potential energy surface and rate coefficients for the global reaction on both doublet and quartet states. We also perform DFT (M06-2X) and CCSD(T) calculations, in order to compare the results. We found that the OPO system possesses a high multiconfigurational character, making DFT and CCSD methodologies not suitable for its potential energy landscape calculation. The rate coefficients have been calculated using the master equation system solver (MESS) package, and the results compared to recent experimental data. It is shown that the quartet state contributes for temperatures higher than 700K. The computed rate coefficient can be described by a modified Arrhenius equation [\(\alpha (T/300)^{\beta } \exp {(-\gamma /T)}\)] with \(\alpha =1.44\times 10^{-12}\text {cm}^{3}\,s^{-1}\), \(\beta =-1.66\) and \(\gamma =704\) K.





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Maciá E (2005) The Role of Phosphorus in Chemical Evolution. Chem Soc Rev 34:691–701
Hunter TP (2012) Why Nature Chose Phosphate to Modify Proteins. Trans R Soc Lond B Biol Sci 367:2513–2516
Goldford JE, Hartman H, Smith TF, Segrè D (2017) Remnants of an Ancient Metabolism without Phosphate. Cell 168:1126–1134
Rivilla VM, Drozdovskaya MN, Altwegg K, Caselli P, Beltran MT, Fontani F, van der Tak FFS, Cesaroni R, Vasyunin A, Rubin M et al (2020) ALMA and ROSINA Detections of Phosphorus-Bearing Molecules: The Interstellar Thread Between Star-Forming Regions and Comets. MNRAS 492:1180–1198. https://doi.org/10.1093/mnras/stz3336
Sousa-Silva C, Seager S, Ranjan S, Petkowski JJ, Zhan Z, Hu R, Bains W (2020) Phosphine as a Biosignature Gas in Exoplanet Atmospheres. Astrobiology 20:235–268
Altwegg K, Balsiger H, Bar-Nun A, Berthelier J-J, Bieler A, Bochsler P, Briois C, Calmonte U, Combi MR, Cottin H et al (2016) Prebiotic chemicals-amino acid and phosphorus|in the coma of comet 67P/Churyumov-Gerasimenko. Sci Adv 2
de la Concepción JG, Puzzarini C, Barone V, Jiménez-Serra I, Roncero O (2021) Formation of phosphorus monoxide (PO) in the interstellar medium: Insights from quantum-chemical and kinetic calculations. Astrophys J 922:169
Jiménez-Serra I, Viti S, Quénard D, Holdship J (2018) The Chemistry of Phosphorusbearing Molecules under Energetic Phenomena. ApJ 862:128
Chantzos J, Rivilla VM, Vasyunin A, Redaelli E, Bizzocchi L, Fontani F, Caselli P (2020) The First Steps of Interstellar Phosphorus Chemistry. A&A 633:A54
Ziurys L, Schmidt D, Bernal J (2018) New circumstellar sources of PO and PN: The increasing role of phosphorus chemistry in oxygen-rich stars. Astrophys J 856:169
Douglas KM, Blitz MA, Mangan TP, Plane JM (2019) Experimental study of the removal of ground-and excited-state phosphorus atoms by atmospherically relevant species. J Phys Che A 123:9469–9478
Douglas KM, Blitz MA, Mangan TP, Western CM, Plane JM (2020) Study of the Reactions PO+ O2 and PO2+ O3 and Spectroscopy of the PO Radical. J Phys Chem A 124:7911–7926
McGuire BA (2021) Census of Interstellar, Circumstellar, Extragalactic, Protoplanetary Disk, and Exoplanetary Molecules. Astrophys J Suppl Ser 259:30
Andreazza C, de Almeida A, Borin A (2016) The radiative association of P and O atoms. Mon Not R Astron Soc 457:3096–3100
Leoch B, Vastel C, Viti S, Jimenez-Serra I, Codella C, Podio L, Ceccarelli C, Mendoza E, Lepine JRD et al (2016) Phosphorus-Bearing Molecules in Solar-Type Star-Forming Regions: First PO Detection. MNRAS 462:3937–3944
Thorne LR, Anicich VG, Prasad SS, Jr WTH (1984) The Chemistry of Phosphorus in Dense Interstellar Clouds. ApJ 280:139–143
Agúndez M, Cernicharo J, Guélin M (2007) Discovery of phosphaethyne (HCP) in space: Phosphorus chemistry in circumstellar envelopes. Astrophys J 662:L91
De Beck E, Kamiński T, Patel N, Young K, Gottlieb C, Menten K, Decin L (2013) PO and PN in the wind of the oxygen-rich AGB star IK Tauri. Astron Astrophys 558:A132
Tenenbaum ED, Woolf NJ, Ziurys LM (2007) Identi cation of Phosphorus Monoxide (X\(^{2}\Pi\)r) in VY Canis Majoris: Detection of the First P-O Bond in Space. ApJ 666:L29–L32
Rivilla VM, Fontani F, Beltrán MT, Vasyunin A, Caselli P, Martín-Pintado J, Cesaroni R (2016) The First Detections of the Key Prebiotic Molecule PO in Star-Forming Regions. ApJ 826:161
Wakelam V, Herbst E, Loison J-C, Smith I, Chandrasekaran V, Pavone B, Adams N, Bacchus-Montabonel M-C, Bergeat A, Béroff K et al (2012) A kinetic database for astrochemistry (KIDA). Astrophys J Suppl Ser 199:21
McElroy D, Walsh C, Markwick A, Cordiner M, Smith K, Millar T (2013) The UMIST database for astrochemistry 2012. Astron Astrophys 550:A36
Manion JA, Huie RE, Levin RD, D RBJr, Orkin VL, Tsang W, Mc- Givern WS, Hudgens JW, Knyazev VD, Atkinson DB et al (2015) NIST Chemical Kinetics Database, NIST Standard Reference Database 17, Version 7.0 (Web Version), Release 1.6.8, Data version 2015.09, National Institute of Standards and Technology, 20899–8320
Husain D, Norris PE (1977) Reactions of phosphorus atoms, P[3p3(4S)3/2], studied by attenuation of atomic resonance radiation in the vacuum ultraviolet. J Chem Soc Faraday Trans 2 73:1107–1115
Husain D, Slater NK (1978) Time-resolved resonance uorescence studies of ground state phosphorus atoms, P[3p3(4S)3/2]. J Chem Soc Faraday Trans 2 74:1627–1643
Clyne MA, Ono Y (1982) Kinetic studies of ground-state phosphorus atoms. J Chem Soc Faraday Trans 2 78:1149–1164
Henshaw T, MacDonald M, Stedman D, Coombe R (1987) The P(4Su)+N3(2Πg) reaction: chemical generation of a new metastable state of PN. J Phys Chem 91:2838–2842
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785
Becke AD (1992) Density-functional thermochemistry. I. The effect of the exchange-only gradient correction. J Chem Phys 96:2155–2160
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98:11623–11627
Zhao Y, Truhlar DG (2008) Exploring the limit of accuracy of the global hybrid meta density functional for main-group thermochemistry, kinetics, and noncovalent interactions. J Chem Theory Comput 4:1849–1868
Peverati R, Truhlar DG (2012) M11-L: A local density functional that provides improved accuracy for electronic structure calculations in chemistry and physics. J Phys Chem Lett 3:117–124
Schmidt MW, Baldridge KK, Boats JA, Elbert ST, Gorgon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S et al (1993) General atomic and molecular electronic structure system. J Comput Chem 14:1347
Werner H-J, Knowles PJ, Knizia G, Manby FR, Schütz M (2012) Molpro: a generalpurpose quantum chemistry program package. WIREs Comput Mol Sci 2:242–253
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140:A1133
Zhao Y, Truhlar DG (2008) Density functionals with broad applicability in chemistry. Accounts of Chemical Research 41:157–167
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241
Dunning THJ Jr (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. Chem Phys 90:1007–1023
Kendall RA, Dunning TH Jr, Harrison RJJ Jr (1992) Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. Chem Phys 96:6796–6806
Mardirossian N, Head-Gordon MJ (2016) How accurate are the Minnesota density functionals for noncovalent interactions, isomerization energies, thermochemistry, and barrier heights involving molecules composed of main-group elements? Chem. Theory Comput 12:4303–4325
Gonzalez C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94:5523–5527
Bartlett RJ (1989) Coupled-cluster approach to molecular structure and spectra: a step toward predictive quantum chemistry. J Phys Chem 93:1697–1708
Bartlett RJ, Watts J, Kucharski S, Noga J (1990) Non-iterative fifth-order triple and quadruple excitation energy corrections in correlated methods. Chem Phys Lett 165:513–522
Raghavachari K, Trucks GW, Pople JA, Head-Gordon M (1989) A fifth-order perturbation comparison of electron correlation theories. Chem Phys Lett 157:479–483
Adler TB, Knizia G, Werner H-J (2007) A simple and efficient CCSD(T)-F12 approximation. J Chem Phys 127: 221106
Knizia G, Adler TB, Werner H-J (2009) Simplied CCSD(T)-F12 methods: Theory and benchmarks. J Chem Phys 130:054104
Szalay PG, Müller T, Gidofalvi G, Lischka H, Shepard R (2012) Multiconfiguration Self-Consistent Field and Multireference Configuration Interaction Methods and Applications. Chem Rev 112:108–181
Bode BM, Gordon MS (1998) MacMolPlt: a graphical user interface for GAMESS. J Mol Graphics Modell 16:133–138
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR (2012) Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminf 4:1–17
Georgievskii Y, Miller JA, Burke MP, Klippenstein SJ (2013) Reformulation and solution of the master equation for multiple-well chemical reactions. J Phys Chem A 117:12146–12154
Eckart C (1930) The penetration of a potential barrier by electrons. Phys Rev 35:1303
Sayós R, Oliva C, Gonzalez M (2002) New analytical (2A’, 4A’) surfaces and theoretical rate constants for the N(4S)+ O2 reaction. J Chem Phys 117:670–679
Caridade PJSB, Varandas AJC (2004) Dynamics Study of the N(4S)+O2 Reaction and Its Reverse. J Phys Chem A 108:3556
San Vicente Veliz JC, Koner D, Schwilk M, Bemish RJ, Meuwly M (2020) The N(4S)+O2(X3Σ)+O(3P)+NO(X2Π) reaction: thermal and vibrational relaxation rates for the 2A’, 4A’and 2A”states. Phys Chem Chem Phys 22:3927–3939
Lee TJ, Taylor PR (1989) A Diagnostic for Determining The Quality of Single-Reference Electron Correlation Methods. Int J Quant Chem Symp 23:199–207
Leininger ML, Nielsen IMB, Crawford TD, Janssen CL (2000) A new diagnostic for open-shell coupled-cluster theory. Chem Phys Lett 328:431–436
Lee TJ (2003) Comparison of the T1 and D1 diagnostics for electronic structure theory: a new definition for the open-shell D1 diagnostic. Chem Phys Lett 372:362–367
Acknowledgements
The authors also acknowledge the National Laboratory for Scientific Computing (LNCC/MCTI, Brazil) for providing HPC resources of the SDumont supercomputer, which have contributed to the research results reported within this paper. URL:http://sdumont.lncc.br, and the Academic Leiden Interdisciplinary Cluster Environment (ALICE) provided by Leiden University. Ahren W. Jasper would like to acknowledge the U. S. Department of Energy, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences, under Contract Number DE-AC02-06CH11357.
Funding
The authors received financial support from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), grant 311508-2021-9, and Fundação de Amparo à Pesquisa do estado de Minas Gerais (FAPEMIG). Rede Mineira de Química (RQ-MG) and CEFET-MG are also acknowledged. Carlos M. R. Rocha received financial support from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement no. 894321. Ahren W. Jasper would like to acknowledge the U. S. Department of Energy, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences, under Contract Number DE-AC02-06CH11357.
Author information
Authors and Affiliations
Contributions
Breno R. L. Galvão designed the research. Data collection and electronic structure calculations were performed by Alexandre C. R. Gomes and Carlos M. R. Rocha. Ahren W. Jasper supervised the kinetics calculations. All authors contributed to the analysis and interpretation. The first draft of the manuscript was written by Alexandre C. R. Gomes and Breno R. L. Galvão and all authors commented, revised and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
This article belongs to the Topical Collection: XXI-Brazilian Symposium of Theoretical Chemistry (SBQT2021)
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gomes, A.C.R., Rocha, C.M.R., Jasper, A.W. et al. Formation of phosphorus monoxide through the \(\mathbf {P}(^{4}S)+\mathbf {O}_{\mathbf {2}}(^{3}\Sigma ^{-})\rightarrow \mathbf {O}(^{3}P)+\mathbf {PO}(^{2}\Pi )\) reaction. J Mol Model 28, 259 (2022). https://doi.org/10.1007/s00894-022-05242-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05242-4