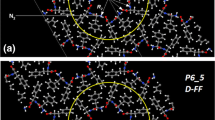
Abstract
The work is devoted to computer studies of the structural and physical properties of such self-organizing structures as peptide nanotubes (PNT) based on diphenylalanine (FF) dipeptide with different initial isomers of the left (L-FF) and right (D-FF) chiralities of these dipeptides. The structures under study are considered both with empty anhydrous and with internal cavities filled with water molecules. Molecular models of both chiralities are investigated using quantum-chemical DFT and semi-empirical methods, which are in consistent with the known experimental data. To study the effect of nano-sized clusters of water molecules embedded in the inner hydrophilic cavity on the properties of nanotubes (including the changes in their dipole moments and polarizations), as well as the changes in the structure and properties of water clusters themselves (their own dipole moments and polarizations), the surfaces of internal cavities of nanotubes and outer surfaces of water cluster structures for both types of chirality are analyzed. A specially developed method of visual differential analysis of structural features of (bio)macromolecular structures is applied for these studies. The results obtained of a number of physical properties (interacting energies, dipole moments, polarization values) are given for various cases and analyzed in comparison with the known data. These data are necessary for analyzing the interactions of water molecules with hydrophilic parts of nanotube molecules based on FF, such as COO- and NH3 + , since they determine many properties of the structures under study. The data obtained are useful for further analysis of the possible adhesion and capture of medical molecular components by active layers of FF-based PNT, which can be designed for creating capsules for targeted delivery of pharmaceuticals and drugs on their basis.




Copyright © 2020, Springer Nature)













Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References s
Calvin M (1969) Chemical evolution. Molecular evolution, towards the origin of living system on the Earth and elsewhere. Claredon, Oxford
Lehninger AL (1972) Biochemistry. The molecular basis of cell structure and function. Worth, New York
Yashima E, Ousaka N, Taura D, Shimomura K, Ikai T, Maeda K (2016) Supramolecular helical systems: helical assemblies of small molecules, foldamers, and polymers with chiral amplification and their functions. Chem Rev 116:13752
Pachahara SK, Subbalakshmi C, Nagaraj R (2017) Formation of nanostructures by peptides. Curr Protein Pept Sci 18(2):1–19
Aryaa SK, Solankia PR, Dattab M, Malhotra BD (2009) Recent advances in self- assembled monolayers based biomolecular electronic devices. J Biosens Bioelectron 24(9):2810–2817
Mendes AC, Baran ET, Reis RL, Azevedo HS (2013) Self-assembly in nature: using the principles of nature to create complex nanobiomaterials. Wiley Interdiscip Rev Nanomed Nanobiotechnol 5(6):582–612
Sharma PP, Rathi B, Rodrigues J (2015) Self-assembled peptide nanoarchitectures: applications and future aspects. Curr Top Med Chem 15:1268–1289. https://doi.org/10.2174/1568026615666150408105711
Quiñones JP, Peniche H, Peniche C (2018) Chitosan based self-assembled nanoparticles in drug delivery. Polymers 10:235. https://doi.org/10.3390/polym10030235
Pauling L, Corey RB (1951) Configurations of polypeptide chains with favored orientations around single bonds. PNAS 37(11):729–740. https://doi.org/10.1073/pnas.37.11.729
Cantor ChR, Schimel PR (1980) Biophysical chemistry. Part 3 The behavior of biological molecules. W.H, San Francisco
Tverdislov VA (2013) Chirality as a primary switch of hierarchical levels in molecular biological systems. Biophysics 58(1):128–132. https://doi.org/10.1134/S0006350913010156
Tverdislov VA, Malyshko EV (2019) On regularities in the spontaneous formation of structural hierarchies in chiral systems of nonliving and living matter. Phys Usp 62(4):354–363. https://doi.org/10.3367/UFNe.2018.08.038401
Bystrov VS, Zelenovskiy PS, Nuraeva AS, Kopyl S, Zhulyabina OA, Tverdislov VA (2019) Chiral peculiar properties of self-organization of diphenylalanine peptide nanotubes:modeling of structure and properties. Math Biol Bioinforma 14(1):94–124. https://doi.org/10.17537/2019.14
Mason SF (1984) Origins of biomolecular handedness. Nature 311:19–23
Chirality and Biological Activity (1990) Eds. Holmstedt B, Frank H, Testa B. Liss, New York
Tishkov VI (2002) Regeneration of cofactors in chiral biosynthesis compounds using degydrogenases. Moscow University Bulletin. Series 2, Chemistry 43 (6):381–388. (in Russian)
Semenova EV, Malyshko EV, Tverdislov VA (2019) On the possible interrelation of the chirality of drugs and chiral structures in target biomacromolecules. Actual Issues Biol Phys Chemi 4(3):346–351 ((in Russian))
Beloglazova IB, Plekhanova OS, Katkova EV et al (2015) Molecular modeling as a new approach to the development of urokinase inhibitors. Bull Exp Biol Med 158(5):700–704. https://doi.org/10.1007/s10517-015-2839-3
Sulimov AV, Kutov DC, Taschilova AS et al (2020) In search of non-covalent inhibitors of SARS-CoV-2 main protease: computer aided drug design using docking and quantum chemistry. Supercomput Front Innov, SsS 7(3):41–56. https://doi.org/10.14529/jsfi200305
Orsi M (2018) Molecular simulation of self-assembly. In: Helena S. Azevedo and Ricardo M.P. da Silva (eds) Self-assembling Biomaterials. 1st Edition. Molecular design, characterization and application in biology and medicine. Woodhead Publishing Series in Biomaterials, Elsevier Ltd., pp. 305–318
Lee OS, Stupp SI, Schatz GC (2011) Atomistic molecular dynamics simulations of peptide amphiphile self-assembly into cylindrical nanofibers. J Am Chem Soc 133(10):3677–3683
Frith WJ (2016) Self-assembly of small peptide amphiphiles, the structures formed and their applications. (A foods and home and personal care perspective). Philos Trans A 374 (2072):2015–0138. https://doi.org/10.1098/rsta.2015.0138
Brandon CJ, Martin BP, McGee KJ, Stewart JJP, Braun-Sand SB (2015) An approach to creating a more realistic working model from a protein data bank entry. J Mol Mod 21(1):11
Ghadiri MR, Granja JR, Milligan RA, McRee DE, Hazanovich N (1993) Self assembling organic nanotubes based on a cyclic peptide architecture. Nature 366:324–332
Görbitz CH (2001) Nanotube formation by hydrophobic dipeptides. Chem Eur J 7:5153–5159
Görbitz CH (2018) Hydrophobic dipeptides: the final piece in the puzzle. Acta Cryst B74:311–318
Bystrov V (2020) Computer simulation nanostructures: bioferroelectric amino acids. Bioferroelectricity: Peptide nanotubes and thymine nucleobase. LAP LAMBERT Academic Publishing
Bystrov VS, Bdikin IK, Singh B (2020) Piezoelectric and ferroelectric properties of various amino acids and tubular dipeptide nanostructures: molecular modelling. Nanomater Sci Eng 2(1):11–24. https://doi.org/10.34624/nmse.v2i1.8259
Sedman VL, Adler-Abramovich L, Allen S, Gazit E, Tendler SJB (2006) Direct observation of the release of phenylalanine from diphenilalanine nanotubes. J Am Chem Soc 128:6903–6908
Scanlon S, Aggeli A (2008) Self-assembling peptide nanotubes. Nano Today 3:22–30
Shklovsky J, Beker P, Amdursky N, Gazit E, Rosenman G (2010) Bioinspired peptide nanotubes: deposition technology and physical properties. Mater Sci Eng B169:62–66
Bystrov VS, Bdikin I, Heredia A, Pullar RC, Mishina E, Sigov A, Kholkin AL (2012) Piezoelectricity and Ferroelectricity in biomaterials: from proteins to self-assembled peptide nanotubes. In: Ciofani G, Menciassi A (eds) Piezoelectric nanomaterials for biomedical applications. Springer, Berlin, pp 187–211
Bystrov VS, Seyedhosseini E, Kopyl S, Bdikin IK, Kholkin AL (2014) Piezoelectricity and ferroelectricity in biomaterials: molecular modeling and piezoresponse force microscopy measurements. J Appl Phys 116(6):066803. https://doi.org/10.1063/1.4891443
Bystrov VS (2016) Computer simulation nanostructures: bioferroelectric peptide nanotubes. LAP Lambert Academic Press, Saarbruecken
Bystrov VS, Paramonova EV, Bdikin IK, Kopyl S, Heredia A, Pullar RC, Kholkin AL (2012) Bioferroelectricity: diphenylalanine peptide nanotubes computational modeling and ferroelectric properties at the nanoscale. Ferroelectrics 440(1):3–24
Nuraeva A, Vasilev S, Vasileva D, Zelenovskiy P, Chezganov D, Esin A, Kopyl S, Romanyuk K, Shur VYA, Kholkin AL (2016) Evaporation-driven crystallization of diphenylalanine microtubes for microelectronic applications. Cryst Growth Des 16:1472–1479
Reches M, Gazit E (2006) Controlled patterning of aligned self-assembled peptide nanotubes. Nat Nanotech 1:195–200
Adler-Abramovich L, Gazit E (2014) The physical properties of supramolecular peptide assemblies: from building block association to technological application. Chem Soc Rev 43:6881–6893
Amdursky N, Molotskii M, Aronov D, Adler-Abramovich L, Gazit E, Rozenman G (2009) Blue luminescence based on quantum confinement at peptide nanotubes. Nano Lett 9(9):3111–3115
Zelenovskiy P, Kornev I, Vasilev S, Kholkin A (2016) On the origin of the great rigidity of self-assembled diphenylalanine nanotubes. Phys Chem Chem Phys 18(43):29681–29685
Zelenovskiy PS, Nuraeva AS, Kopyl S, Arkhipov SG, Vasilev SG, Bystrov VS, Gruzdev DA, Waliszek M, Svitlyk V, Shur VYA, Marfa L, Kholkin AL (2019) Chirality-dependent growth of self-assembled diphenylalanine microtubes. Cryst Growth Des 19:6414–6421. https://doi.org/10.1021/acs.cgd.9b00884
Bystrov VS, Kopyl SA, Zelenovskiy P, Zhulyabina OA, Tverdislov VA, Salehli F, Ghermani NE, Shur VYA, Kholkin AL (2018) Investigation of physical properties of diphenylalanine peptide nanotubes having different chiralities and embedded water molecules. Ferroelectrics 525:168–177. https://doi.org/10.1080/00150193.2018.14328
Bystrov VS, Zelenovskiy PS, Nuraeva AS, Kopyl S, Zhulyabina OA, Tverdislov VA (2019) Molecular modeling and computational study of the chiral-dependent structures and properties of the self-assembling diphenylalanine peptide nanotubes. J Mol Model 25:199. https://doi.org/10.1007/s00894-019-4080-x
Bystrov VS, Coutinho J, Zelenovskiy PS, Nuraeva AS, Kopyl S, Filippov SV, Zhulyabina OA, Tverdislov VA (2020) Molecular modeling and computational study of the chiral-dependent structures and properties of the self-assembling diphenylalanine peptide nanotubes, containing water molecules. J Mol Model 26(11):326. https://doi.org/10.1007/s00894-020-04564-5
Bystrov V, Coutinho J, Zelenovskiy P, Nuraeva A, Kopyl S, Zhulyabina O, Tverdislov V (2020) Structures and properties of the self-assembling diphenylalanine peptide nanotubes containing water molecules: modeling and data analysis. Nanomaterials 10(10):1999. https://doi.org/10.3390/nano10101999
Bystrov VS, Coutinho J, Zhulyabina OA, Kopyl SA, Zelenovskiy PS, Nuraeva AS, Tverdislov VA, Filippov SV, Kholkin AL, Shur VYA (2021) Modelling and physical properties of diphenylalanine peptide nanotubes containing water molecules. Ferroelectrics 574:78–91. https://doi.org/10.1080/00150193.2021.1888051
Emtiazi G, Zohrabi T, Lee LY, Habibi N, Zarrabi A (2017) Covalent diphenylalanine peptide nanotube conjugated to folic acid/magnetic nanoparticles for anti-cancer drug delivery. J Drug Deliv Sci Technol 41:90–98. https://doi.org/10.1016/j.jddst.2017.06.005
Silva RF, Araújo DR, Silva ER, Ando RA, Alves WA (2013) L-Diphenylalanine microtubes as a potential drug-delivery system: characterization, release kinetics, and cytotoxicity. Langmuir 29:10205–10212. https://doi.org/10.1021/la4019162
Filippov SV, Bystrov VS (2020) A visual differential analysis of structural features of internal cavities in two chiral forms of diphenylalanine nanotubes. Biophysics 65(3):374–380. https://doi.org/10.1134/S0006350920030057
Filippov SV, Likhachev IV, Bystrov VS (2020) Visual-differential analysis of structural realignations water clusters in the domain of the D-FF nanotubes. Russ J Biol Phys Chem 5(3):415–423
Bystrov VS, Filippov SV, Zhulyabina OA, Tverdislov VA (2020) Molecular modeling of the structure and properties of diphenylalanine peptide nanotubes of different chirality containing water molecules. Russ J Biol Phys Chem 5(2):261–268
The Cambridge Crystallographic Data Centre (CCDC). https://www.ccdc.cam.ac.uk/ (accessed July 2018–May 2020) Crystallographic data for D-FF nanotubes structure reported in [41, 43] have been deposited in the Cambridge Crystallographic Cambridge Crystallographic Data Centre, no. CCDC 1853771. For L-FF crystallographic data no. CCDC 16337 was deposited earlier (as reported in [25])
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140:A1133
VASP (Vienna Ab initio Simulation Package). https://www.vasp.at/ (Accessed July 2019–May 2020)
Kresse G, Hafner J (1994) Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys Rev B 49:14251–14269. https://doi.org/10.1103/PhysRevB.49.14251
Kresse G, Furthmüller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B: Condens Matter Mater Phys 54:11169–11186. https://doi.org/10.1103/PhysRevB.54.11169
Kresse G, Joubert D (1999) From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B: Condens Matter Mater Phys 59:1758–1775
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Paier J, Hirschl R, Marsman M, Kresse G (2005) The Perdew-Burke-Ernzerhof exchange-correlation functional applied to the G2–1 test set using a plane-wave basis set. J Chem Phys 122:234102
Grimme S, Antony J, Ehrlich S, Krieg S (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (dft-d) for the 94 elements H-Pu. J Chem Phys 132:154104
Stewart JJP (1989) Optimization of parameters for semiempirical methods. I Method J Comput Chem 10:209
Stewart JJP (1989) Optimization of parameters for semiempirical methods II. Applications. J Comput Chem 10:221
Stewart JJP (2007) Optimization of parameters for semiempirical methods V: modification of NDDO approximations and application to 70 elements. J Mol Mod 13(12):1173–1213
Rocha GB, Freire RO, Simas AM, Stewart JJP (2006) RM1: a Reparameterization of AM1 for Y, C, N, O, P, S, F, Cl, Br, and I. J Comp Chem 27(10):1101–1111
Lima NBD, Rocha GB, Freire RO, Simas AM (2019) RM1 semiempirical model: chemistry, pharmaceutical research, molecular biology and materials science. J Braz Chem Soc 30(4):683–716. https://doi.org/10.21577/0103-5053.20180239
Hypercube Inc (2011) HyperChem 8. Tools for Molecular Modeling. Professional Edition For Windows AC Release 8.0 USB (on CD). Hypercube Inc., Gainesville
Novotny M, Kleywegt GJ (2005) A survey of left-handed helices in protein structures. J Mol Biol 347(2):231–410. https://doi.org/10.1016/j.jmb.2005.01.037
Gremer L, et al. (2017) Fibril structure of amyloid-b(1–42) by cryo–electron microscopy. Science 358:116–119. http://science.sciencemag.org/content/358/6359/116
Andrade-Filho T, Martins TC, Ferreira FF, Alves WA, Rocha AR (2016) Water-driven stabilization of diphenylalanine nanotube structures. Theor Chem Acc 135:185. https://doi.org/10.1007/s00214-016-1936-3
O’Boyle NM, Banck M, James CA et al (2011) Open Babel: an open chemical toolbox. J Cheminform 3:33. https://doi.org/10.1186/1758-2946-3-33
Blender is the free and open source 3D creation suite. It supports the entirety of the 3D pipeline —modeling, rigging, animation, simulation, rendering, compositing and motion tracking, even video editing and game creation. https://www.blender.org (accessed 05.04.2021)
Filippov SV, Sivozhelezov VS (2018) Method of constructing dynamic molecular models within the environment of the Blender open 3D platform exemplified by β2-adrenergic receptor. In: Lakhno VD (eds) Proceedings of the International Conference “Mathematical Biology and Bioinformatics”. Vol. 7. IMPB RAS, Pushchino; paper No. e45. https://doi.org/10.17537/icmbb18.23
Filippov SV (2018) Methods of working with dynamic molecular models, built in an environment of open 3D editor Blender. In: Lakhno VD (eds) Proceedings of the International Conference “Mathematical Biology and Bioinformatics”. Vol. 7. IMPB RAS, Pushchino; paper No. e43. https://doi.org/10.17537/icmbb18.62
Filippov SV (2019) Visualization of macromolecules in 3D-editors: a method for identifying atoms on images. In: Proceedings of the International Conference after A.F. Terpugov (June, 26–30, Saratov, Russia): Information Technologies and Mathematical modelling (ITMM-2019). Publishing Sci.-Techn.Lit., Tomsk, Vol.1, pp.169–174 (in Russian)
Filippov SV, Polozov RV, Sivozhelezov VS (2019) Visualization of spatial structures of (bio) macromolecules: “hypsometric” maps construction. In: Proceedings of the International Conference after A.F. Terpugov (June, 26–30, Saratov, Russia): information technologies and mathematical modelling (ITMM-2019). Publishing Sci.-Techn.Lit., Tomsk, Vol.1, pp.163–168 (in Russian)
Filippov SV, Polozov RV, Sivozhelezov VS (2019) Hypsometric mapping based visualization of (bio)macromolecular 3D structures. KIAM Preprint 61, Moscow, 2019. pages 14. https://doi.org/10.20948/prepr-2019-61. URL: http://library.keldysh.ru/preprint.asp?id=2019-61 (in Russian)
Filippov SV, Polozov RV, Sivozhelezov VS (2019) “Hypsometric” maps of spatial molecular structures. In: Abstracts of International Conference «Advanced Mathematics, Computations and Applications 2019» (AMCA-2019), (July, 1–5, Novosibirsk, Russia). IPC NSU, Novosibirsk, 167 pages. https://doi.org/10.24411/9999-017A-2019-10324 (in Russian)
Filippov SV (2019) Projection "hypsometric" maps of molecular structures, Blender 3D editor: Identification of atoms. In: Abstracts of International Conference «Advanced Mathematics,Computations and Applications 2019» (AMCA-2019), (July, 1–5, Novosibirsk, Russia). IPC NSU, Novosibirsk, 167 pages. https://doi.org/10.24411/9999-017A-2019-10323 (in Russian)
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675
LibreOffice. URL: https://www.libreoffice.org (Accessed 07.04.2021)
Tschumperle D, Fourey S. G’MIC (GREYC’s Magic for Image Computing): a full-featured open-source framework for image processing. URL: https://gmic.eu (Accessed 07.04.2021)
Photo Reactor is a Nodal Image Processor. URL: https://www.mediachance.com/reactor/index.html (Accessed 07.04.2021)
Salehli F, Aydin AO, Chovan D, Kopyl S, Bystrov V, Thompson D, Tofail SAM, Kholkin A (2021) Nanoconfined water governs polarization-related properties of self-assembled peptide nanotubes. Nano Select 2:817–829. https://doi.org/10.1002/nano.202000220
Acknowledgements
The authors express their gratitude to V.A. Tverdislov for fruitful discussions and support of this line of research. The authors are grateful to the University of Aveiro (UA, Faculty of Physics), Portugal, for the opportunity to use a multi-processor computer cluster for computational research on this study within the framework of non-commercial scientific agreement between IMPB RAS (the branch of KIAM RAS) and UA for the period 2017–2020 of our collaboration. In addition, the authors thank the Center for Collective Use of the Keldysh Institute of Applied Mathematics RAS for the possibility of the use of a hybrid supercomputer.
Author information
Authors and Affiliations
Contributions
V.B. wrote the manuscript and supervised this study. S.F. developed the method of the visual differential analysis and applied it to the FF nanotubes and water clusters.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bystrov, V.S., Filippov, S.V. Molecular modelling and computational studies of peptide diphenylalanine nanotubes, containing waters: structural and interactions analysis. J Mol Model 28, 81 (2022). https://doi.org/10.1007/s00894-022-05074-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05074-2