Abstract
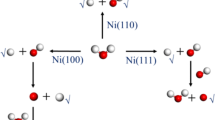
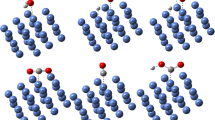
This work has presented a calculated study of the water–gas shift reaction (WGSR) performing on the models of ZnO \(\left(10\overline{1 }0\right)\) only and six-atomic copper cluster deposited on the ZnO surfaces (6Cu/ZnO) using density functional theory (DFT). The most stable configurations of ZnO and 6Cu/ZnO surfaces were found and used for the mechanism calculations of WGSR. The carboxyl mechanism of WGSR was proposed to find the reaction pathway. Based on this pathway, WGSR occurred at the elementary reaction of COOH intermediate formation as the rate-controlling step on 6Cu/ZnO surface, and the elementary reaction of H–H association as the rate-controlling step on ZnO surface, in which the highest activation energies were calculated as 1.05 eV and 1.56 eV for 6Cu/ZnO and ZnO surfaces, respectively. These calculations indicated that the 6Cu/ZnO was more favorable and more effective than ZnO as a catalyst for WGSR. In addition, the nature of bonds of CO and H2O adsorption on ZnO and 6Cu/ZnO surfaces was also analyzed using the local density of states (LDOS) and electron density difference (EDD) methods.
Graphical abstract







Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Code availability
Vienna ab initio Simulation Package (VASP) program.
References
Haryanto A, Fernando S, Murall N, Adhikari S (2005) Current status of hydrogen production techniques by steam reforming of ethanol: a review. Energy Fuels 19:2098–2106
Wilson JR, Burgh G (2008) Energizing our future: rational choices for the 21st century. Wiley, Hoboken, NJ
Holladay JD, Wang Y, Jones E (2004) Review of developments in portable hydrogen production using microreactor technology. Chem Rev 104:4767–4790
Olah GA, Goeppert A, Prakash GKS (2006) Beyond oil and gas: the methanol economy. Wiley-VCH, Weinheim
Lee Y-K, Kim K-S, Ahn J-G, Son I-H, Shin WC (2010) Hydrogen production from ethanol over Co/ZnO catalyst in a multi-layered reformer. Int J of Hydrogen Energy 35:1147–1151
Peng SF, Ho JJ (2011) The mechanism of the water-gas shift reaction on Cu/TiO2(110) elucidated from application of density-functional theory. Phys Chem Chem Phys 13:20393–20400
Tang QL, Chen ZX, He X (2009) A theoretical study of the water gas shift reaction mechanism on Cu(1 1 1) model system. Surf Sci 603:2138
Rodríguez JA, Liu P, Hrbek J, Perez M, Evans J (2008) Water–gas shift activity of Au and Cu nanoparticles supported on molybdenum oxides. J Mol Catal A 281:59
Gokhale AA, Dumesic JA, Mavrikakis M (2008) On the mechanism of low-temperature water gas shift reaction on copper. J Am Chem Soc 130:1402
Shishido T, Yamamoto M, Li D, Tian Y, Morioka H, Honda M, Sano T, Takehira K (2006) Water-gas shift reaction over Cu/ZnO and Cu/ZnO/Al2O3 catalysts prepared by homogeneous precipitation. Appl Catal A: General 303:62–71
Cong VT, Huynh LK, Jiang J-C, Nhung NTA, Tat PV (2016) Density function theory study of water gas shift reaction on 2Cu/ZnO (10–10) surface. Comput Theor Chem 1081:62–70
Huang S, Lin C, Wang JH (2010) Trends of water gas shift reaction on close-packed transition metal surfaces. J Phys Chem C 114:9826–9834
Harrison AWaNM (2001) An ab initio study of hydrogen adsorption on ZnO(101h0), J. Phys Chem B, 105, 6191–6193.
Wander NMHA (2003) The structure of higher defective ZnO(101–0). Surf Sci 529:L281–L284
Greenwood NN, Earnshaw A (1997) Chemistry of the elements, 2nd ed., Elsevier
Meyer B, Marx D (2003) Density-functional study of the structure and stability of ZnO surfaces. Phys Rev Lett B 67:035403
Wöll C (2007) The chemistry and physics of zinc oxide surfaces. Prog Surf Sci 82:55–120
Pala RGS, Metiu H (2008) Selective promotion of different modes of methanol adsorption via the cation substitutional doping of a ZnO(10.10) surface. J Catal 254:325–331
Diebold U, Koplitz LV, Dulub O (2004) Atomic-scale properties of low-index ZnO surfaces. Appl Surf Sci 237:336–342
Hu J, Guo W-P, Shi X-R, Li B-R, Wang J (2009) J Phys Chem C 113:7227–7235
Zhibo Ren FP, Chen B, Mei D, Li J (2017) A combined experimental and computational study of water-gas shift reaction over rod-shaped Ce0.75M0.25O2 (M¼Ti, Zr, and Mn) supported Cu catalysts. International Journal of Hydrogen Energy, 1–12
Rodriguez JA, Liu P, Wang X, Wen W, Hanson J, Hrbek J, Perez M, Evans J (2009) Water-gas shift activity of Cu surfaces and Cu nanoparticles supported on metal oxides(experimental). Catal Today 143:45
Tang QL, Liu ZP (2010) Identification of the active Cu phase in the water-gas shift reaction over Cu/ZrO2 from first principles. J Phys Chem C 114:8423
Michael Estrella LB, Zhou Gong, Wang Xianqin, Wang Qi, Wen Wen, Hanson Jonathan C, Frenkel Anatoly I, Rodriguez José A (2009) In situ characterization of CuFe2O4 and Cu/Fe3O4 water-gas shift catalysts. J Phys Chem C 113:14411–14417
Shin Ichiro Fujita MU, Takezawa N (1992) Mechanism of the reverse water gas shift reaction over Cu/ZnO catalyst. J Catal 134, 220.
Kuang X, Wanga X-Q, Liu G-B (2011) A density functional study on the adsorption of hydrogen molecule onto small copper clusters, Journal of. Chem Sci 123:743–754
Chen W-K, Liu S-H, Cao M-J, Yan Q-G, Lu C-H (2006) Adsorption and dissociation of methanol on Au(1 1 1) surface: a first-principles periodic density functional study. J Mol Struct (Thoechem) 770:87–91
Harrison NM (2003) An introduction to density functional theory, Computational Materials Science, NATO Science Series III. Ed Catlow and Kotomin 187:1–26
Vo CT, Huynh LK, Hung J-Y, Jiang J-C (2013) Methanol adsorption and decomposition on ZnO(1010) surface: a density functional theory study. Applied Surface Science 280:219–224
Yun Zhao BT, Yang Zongxian, Zhao Yue, Zhao Leihong, Luo Mengfei (2011) Density functional theory study of Sn adsorption on the CeO2 surface. J. Phys. Chem. C 115:16461–16466
Jaffe JE, Snyder JA, Lin Z, Hess AC (2000) LDA and GGA calculations for high-pressure phase transitions in ZnO and MgO. Phys Rev Lett B 62:3
Kresse G, Furthmüller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Physical Review B 54:16
Kresse G, Hafner J (1993) Ab initio molecular dynamics for liquid metals. Phys Rev B 47:1
Kresse G, Hafner J (1993) Ab initio molecular dynamics for open-shell transition metals. Phys Rev B 48:17
Kresse G, Joubert D (1999) From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B 59:3
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1992) Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys Rev B 46:6671
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1992) Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys Rev B 46:6671
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1993) Erratum: Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys Rev B 48:4978
Jonsson H, Mills G, Jacobsen KW (1998) Nudged elastic band method for finding minimum energy paths of transition, In Classical and quantum dynamics in condensed phase simulations, Ed. B. J. Berne, G. Ciccotti and D. F. Coker, World Scientific, 385
Henkelman G, Jónsson H (2000) Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J Chem. Phys 113:9978
Graeme Henkelman BPU, Jónsson Hannes (2000) A climbing image nudged elastic band method for finding saddle points and minimum energy paths. Journal OF Chemical Physics 113:22
Hung J-Y, Jiang J-C (2009) Density functional theory study of water gas shift reaction on ZnO(1010) and Pd/ZnO(1010) surfaces in, 1–90
Hung Y-L (2010) DFT study of methanol decomposition and water dissociation on Pd/ZnO(1010) surface
Huang S-C, Lin C-H, Wang JH (2010) Trends of water gas shift reaction on close-packed transition metal surfaces. The Journal of Physical Chemistry C 114:9826–9834
Monkhorst HJ, Pack JD (1976) Phys Rev B 13:5188
Shi X-R, Wang S-G, Hu J, Wang H, Chen Y-Y, Qin Z, Wang J (2009) Density functional theory study on water–gas-shift reaction over molybdenum disulfide. Appl Catal A 365:62
Lehninger AL, Nelson DL, Cox MM (2005) Lehninger principles of biochemistry, 4 ed., W.H.Freeman
Cottrell TL (2005) The strengths of chemical bonds, 2nd edn. Butterworths, London
Acknowledgements
We are also grateful to the National Center of High-Performance Computing (NCHC) for donating computer time and facilities.
Funding
This work was financed by the Industrial University of Ho Chi Minh City in the project with the core of contractual 21/1H06.
Author information
Authors and Affiliations
Contributions
Dr. Vo Thanh Cong: supervision, conceptualization, methodology, software, data analyses, writing the original draft, review and editing. Dr. Nguyen Van Son: review and editing. Dr. Do Quy Diem: review and editing. Dr. Son Quynh Thai Pham: supervision, data analyses, writing, finishing the final manuscript, review and editing.
Corresponding authors
Ethics declarations
Ethics approval
Our manuscript has not been submitted to more than one journal for simultaneous consideration.
Our manuscript has not been published previously (partly or in full).
A single study is not split up into several parts to increase the quantity of submissions and submitted to various journals or to one journal over time (e.g., “salami-publishing”).
No data have been fabricated or manipulated (including images) to support our conclusions.
No data, text, or theories by others are presented as if they were the author’s own (“plagiarism”).
Consent to participate
Our current study did not involve human subject and has not used living animals, etc.
Consent for publication
The authors agree to publish this work on the Journal of Molecular Modeling.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cong, V.T., Van Son, N., Diem, D.Q. et al. A comparison of water–gas shift reaction on ZnO \(\left(10\overline{1 }0\right)\) surface and 6Cu cluster deposited over ZnO \(\left(10\overline{1 }0\right)\) surface using density functional theory studies. J Mol Model 28, 84 (2022). https://doi.org/10.1007/s00894-022-05057-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05057-3